Ever since the first bispecific antibody received regulatory approval in 2009, increasingly sophisticated bispecific antibodies have been sought, particularly in cancer research. Bispecific antibodies now represent one of the livelier fields in cancer immunotherapy. Indeed, advances in bispecific antibodies were the subject of a day-and-a-half program at the 10th Annual Immuno-Oncology Summit.
The event’s organizer, the Cambridge Healthtech Institute, promised to showcase novel targeting approaches, new constructs, and ongoing efforts to widen the range of target antigens. Some of the event’s most interesting presentations are revisited here. They address topics such as the development of bispecific antibodies that approach familiar targets (such as CD3) in new ways; the use of a dual-specific inhibitor for the simultaneous targeting of two immune checkpoint ligands (PD-L1 and PD-L2); the selective depletion of regulatory T cells to concentrate immunosuppression on solid tumors; and the recruitment of immune cells other than T cells into the tumor microenvironment.
Hot and cold
In 2009, at the behest of the European Medicines Administration, catumaxomab (Removab) became the world’s first bispecific antibody to be approved for therapeutic use. It was trifunctional, binding tumor cells via the EpCAM antigen, T cells via the CD3 protein complex, and accessory cells (such as macrophages, natural killer cells, or dendritic cells) via the Fc receptor.
Although catumaxomab was subsequently withdrawn from the market for commercial reasons, it has been succeeded by other bispecific antibodies that incorporate CD3-targeting structures and are intended to treat cancer. For example, bispecific antibodies that target CD3 and tumor surface receptors are being developed by ACROBiosystems, a biotechnology company based in Newark, DE. Each of these bispecific antibodies is designed to bring together a T cell and a tumor cell to form an immunological synapse.
At the Immuno-Oncology Summit, ACROBiosystems was represented by Teng Peng, PhD, who was then (but is no longer) one of the company’s technical application managers. She asserted that the company’s bispecific antibodies offer high affinity and high efficacy. She added that the company’s bispecific antibodies are designed to limit off-target effects, which represent an ongoing challenge for CD3 technologies.
After the event, GEN followed up with Tracy Zhao, a field application scientist at ACROBiosystems. She noted that although ACROBiosystems has provided CD3 bispecific antibodies as “research use only” products, typically for the development and screening of drug candidates, the company recently established a GMP facility that supports the production of antibodies for human use. “Soon, it will be easier for our customers to choose our GMP products for use in clinical studies,” she said.
The most successful immunotherapies have focused on “hot” tumors—that is, tumors in which pro-inflammatory factors are present and serve to promote T-cell infiltration and stronger immune responses. Such therapies include those incorporating CD3 bispecific antibodies and checkpoint inhibitors.
PD-1 and PD-L1 checkpoint inhibitors have exploded in popularity over the last decade due to their ability to reinvigorate T cells that are otherwise suppressed by tumors. These inhibitors, however, have proven less useful in treating “cold” tumors—those that suppress the immune response by excluding T cells, thereby negating the mechanism underlying checkpoint inhibitors. Such tumors make up the majority of cancers, affecting five times as many patients.
Michael Curran, PhD, an immunologist at the MD Anderson Cancer Center and the founder of ImmunoGenesis, a clinical-stage biotechnology company based in Houston, TX, presented data on the company’s dual-specific antibody, IMGS-001. According to Curran, IMGS-001 augurs a “second generation” of PD-1 therapies. Rather than targeting only PD-1 or PD-L1, ImmunoGenesis’s antibody binds to both PD-L1 and the second PD-1 ligand, PD-L2.
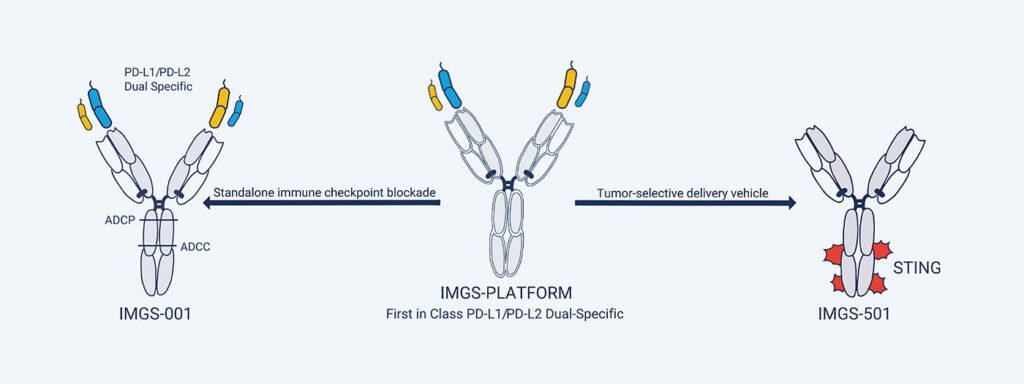
Curran noted that over the last five to seven years, scientists have found that PD-L2 binds PD-1 with greater affinity than does PD-L1, and that PD-L2 is also expressed widely across many cancer types, including some that don’t express PD-L1. (About 60% of cancers express both L1 and L2; about 15%, just L1; and about 15%, just L2—the remaining 10% express neither.) He added that scientists also came to realize that PD-L2’s biology is complex. The latter finding has proven discouraging. As Curran remarked, “No one has really felt they understood [PD-L2] enough to try to target it directly.”
But in creating a dual-specific antibody that can bind either or both ligands, IMGS-001 functions as a checkpoint inhibitor while also depleting other tumor-associated cell types, including myeloid stroma and myofibroblasts that together create a physical barrier that excludes T cells. In addition, the antibody reconditions the endothelial vasculature supplying blood to the tumor.
“The antibody allows an influx of T cells into these cold tumors that otherwise are not well infiltrated,” Curran summarized. “The PD-1-blocking function of the antibody then allows them to amplify, and we see dramatically elevated responses in cold tumor settings compared to the existing drugs.” In preclinical models that tested the drug as a monotherapy and in which existing PD-1 antibodies have been largely ineffective, IMGS-001 cured roughly 50% of mice and slowed tumor growth by over 90%. ImmunoGenesis is expected to begin dosing patients in a Phase I trial this month.
Leveraging new platforms
Also on display were new technologies that yield more consistent and powerful products than past iterations of bispecific antibodies. Early attempts at producing these antibodies were challenged by the fact that bispecific antibodies can assume many conformations, only some of which are the desired product.
Daniel Pereira, PhD, the CSO at Invenra, a biotechnology company based in Madison, WI, walked attendees through preclinical data related to INV322, a bispecific antibody for the selective depletion of T regulatory cells (Tregs). INV322 co-targets CD25 and CTLA-4 to reduce immunosuppression in solid tumors.
Invenra developed INV322 using the B-Body and SNIPER platforms, which together optimize affinities for both antigens to take advantage of avidity and better targeting of CD25/CTLA-4 co-expressing regulatory T cells in the tumor microenvironment. Together, the platforms produce a bispecific antibody that assumes the correct conformation up to 85% of the time. “In the bispecific field,” Pereira asserted, “that’s among the best that we’ve seen.”
A preclinical murine model of colon cancer showed that a surrogate antibody prompts durable tumor regression after a single low dose and also invokes long-lasting memory against subsequent challenges with the same tumor. It also skewed the response toward Tregs in the tumor microenvironment, rather than a global response—a result directly attributed to the SNIPER platform, which produces antibodies with strong avidity to tumor Tregs coexpressing both CD25 and CTLA-4.
Looking forward, Pereira says that INV322 could be a good candidate to couple with existing PD-1/PD-L1 therapies in hot tumors. “In addition, Tregs are present in higher numbers in cold tumors where the checkpoint inhibitors haven’t done so well,” Pereira noted. “We’ve seen through other programs that are working on Treg depletion that they seem to be forging ahead in these colder tumors, so we would expect with our added skewing in the tumor microenvironment, we’d do well there, too.”
Moving beyond T cells
Hui Zou, PhD, the CSO at Phanes Therapeutics, shared data on a new bispecific antibody that targets CD47 and tumor-associated antigens (TAAs) to reactivate other cell types—specifically, macrophages and natural killer cells—that are present but suppressed in the tumor microenvironment. At Phanes, scientists were aware that CD47 had long been a popular molecular target due to its overexpression on many cancer cells. Accordingly, when these scientists first considered targeting CD47, they wondered, Zou recalled, whether the company was “arriving late to the party.”

But one persistent issue with CD47-based monospecific antibody therapies is the presence of off-target effects. These off-target effects are induced, in part, because CD47 occurs not only on cancer cells, but also on many normal cells, particularly red blood cells. “When you target CD47,” Zou said, “the adverse effect is anemia” and thrombocytopenia, a decrease in the amount of blood platelets.
Zou related how Phanes leveraged its proprietary PACbody and SPECpair platforms to design bispecific antibodies that “create a new biology.” Essentially, the company began generating bispecific antibodies that could target two receptors on a cancer cell. Bispecific antibodies with this ability are more likely to bind only to cancer cells and not to red blood cells.
In addition, the platforms assemble bispecific antibodies that incorporate native immunoglobulin G-like structures. These bispecific antibodies activate not just the innate immune system via natural killer cells and macrophages, but the adaptive immune system as well. This makes for a more flexible approach. When attacking a tumor, the bispecific antibodies can induce both antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis.
Phanes has since dosed the first patient in a Phase I trial for its PT886 bispecific antibody, which targets CD47 and the protein claudin 18.2 in pancreatic, gastric, and esophageal cancers. An anti-DLL3/anti-CD47 bispecific antibody, PT217, has received FDA clearance to proceed with a clinical trial treating small cell lung cancer.
Although the scientists at Phanes initially feared that they might be getting a late start, the company’s recent successes show that there are still opportunities to develop bispecific antibodies that can advance cancer immunotherapy. “Bispecific antibodies allow scientists and drug developers to be a lot more creative,” Zou declared. “You can think about completely new ways of attacking the tumor cells with some really novel bispecific designs, and I think that’s really the future of antibody-based therapy.”

