SAN DIEGO, CA—For the first time, one-year-old company EpiBiologics is showing proof-of-concept data from their platform for targeted degradation of membrane and extracellular proteins—a treasure trove that has long eluded drug developers.

“We’re aiming protein degradation into the extracellular space, and that’s about 40% of the proteome,” said CEO and president Ann Lee-Karlon, PhD, a former Genentech executive and chief operating officer of Altos Labs, who joined EpiBiologics in San Mateo, California, in July 2023. “I love that because [EpiTACs are] bispecific antibody-based, there’s a clear path if you can de-risk the pharmacology.”
The platform is based on a modular bispecific antibody-based system called EpiTACs, developed by EpiBiologics’ co-founder and renowned antibody engineer, Jim Wells, PhD, of the University of California, San Francisco (UCSF). EpiTACs enable the degradation of disease-driving extracellular and membrane proteins in a tissue-specific manner. One arm of the bispecific antibody goes for the target, and the other arm can be tissue or disease-specific, sparing normal tissue and driving up the therapeutic index.
The company launched in March 2023 with an initial $50 million in Series A funding co-led by Mubadala Capital and Polaris Partners, with participation from Vivo Capital and GV. That funding round eventually grew to over $70 million with contributions from Digitalis Ventures, Taiho Ventures, and Codon Capital.
A trio of degraders
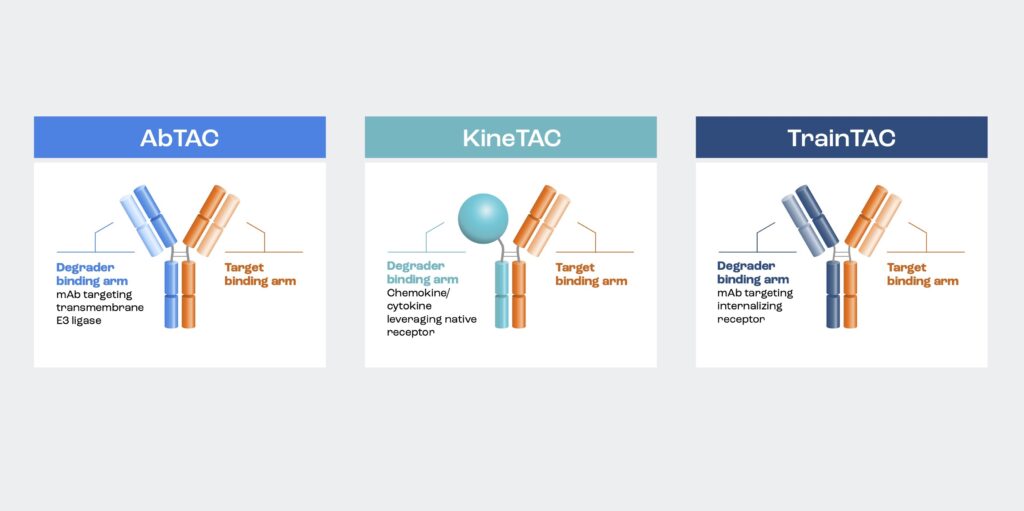
EpiBiologics has a catalog of more than 270 degrader arms for their EpiTACs, which fall into three categories: AbTACs, KineTACs, and TrainTACs.
AbTACs facilitate the interaction between the protein of interest and transmembrane E3 ligases, enabling the ubiquitination and degradation of membrane proteins by the proteasome. KineTACs exploit the inherent internalization mechanism facilitated by chemokine or cytokine receptors, whereby they bind to the receptor through the native ligand to induce internalization and lysosomal degradation of both soluble and membrane targets. And TrainTACs use a variety of internalizing receptors to transport membrane and soluble extracellular proteins for lysosomal degradation.
Mutation-independent receptor targeting
Chief scientific officer Shyra Gardai, PhD, said that the data is derived from efforts to drug the epidermal growth factor receptor (EGFR), a well-known cancer target that has therapeutic limitations because of on-target toxicity in the skin and resistance mechanisms that arise.

“That’s a nice example where we can have tissue-specific degraders to limit that on-target toxicity in normal tissue,” said Gardai. “We’re also able to limit the downstream signaling that EGFR can drive through degradation, which you don’t see with just simple blockade of EGFR.”
Another highlight for EpiBiologics at AACR is the data demonstrating that the activity of the EpiTACs is independent of EGFR mutational status.
“This leverages the types of targets that we can go after that could have these different mutational forms because EpiTACs can grab the outside and drive degradation—the intracellular form doesn’t matter,” said Gardai.
According to Lee-Karlon, EGFR is a good proof-of-concept, but the company is also looking at ion channels or G-protein-coupled receptors (GPCRs) that are hard to target with small molecules.
“Shyra and the team have built and are testing over 1,000 [EpiTACs],” said Lee-Karlon. “So, we know [the platform] is scalable and manufacturable.”
From proof-of-concept to pre-IND
This year, the priority for EpiBiologics is to think in terms of developmental candidates. According to Gardai, an advantage of the EpiTac platform is the speed from which they can go from initial studies to in vivo proof-of-concept and see if they have a clinical opportunity.
“The data we’re highlighting is basically a year’s worth of work from that screening to in vivo proof-of-concept, standard-of-care benchmarking and demonstrating that we have a survival benefit,” said Gardai.
For Lee-Karlon, the goal is to nominate a candidate to file an investigational new drug (IND) application by the end of the year. “We’re headed toward a development candidate selection this year—that’s our top priority,” said Lee-Karlon. “If we’re fortunate, we might even have more than one. We are already working in parallel to make sure all activities are lining up appropriately at the right time.”
From there, the race begins to cross the finish line of approval, one that Lee-Karlon knows well, having done so with Ocrevus for multiple sclerosis and Rituxan in immunology.


