Source: Repligen
A conventional production fed-batch process involves a multi-stage seed expansion and elaborate clarification steps to isolate the protein of interest from other process impurities. The current clarification technology utilizes centrifugation and depth filtration trains that are complex and limited in their ability to operate in a sterile or low-bioburden manner. With an increase in demand for production of protein therapeutics, it is essential to simplify the traditional fed-batch processes to result in better yield, shorter time, and smaller footprint.
Repligen developed an intensified fed-batch process using XCell™ ATF technology (Figure 1). This process involves inoculating the fed-batch bioreactor at high seeding density followed by a novel clarification method, High Productivity Harvest (HPH), which helps maximize the yield of a fed-batch bioreactor in half the duration while eliminating centrifugation, depth, and sterile filtration steps.
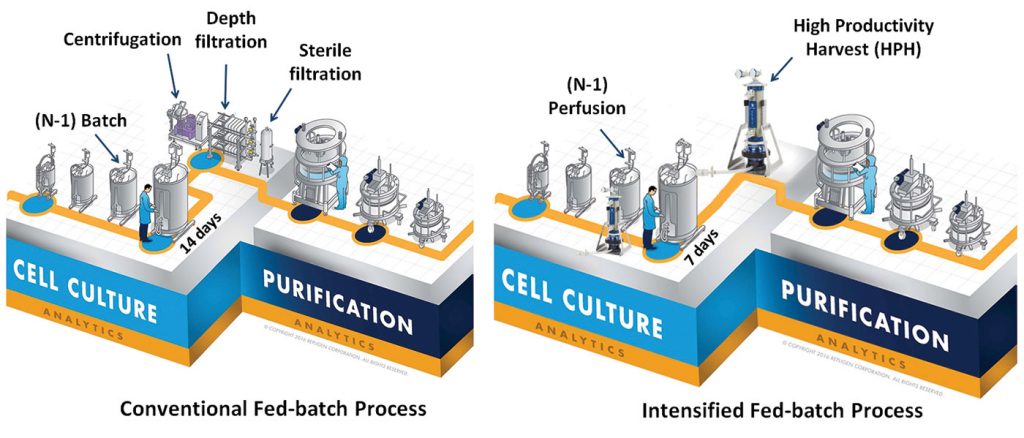
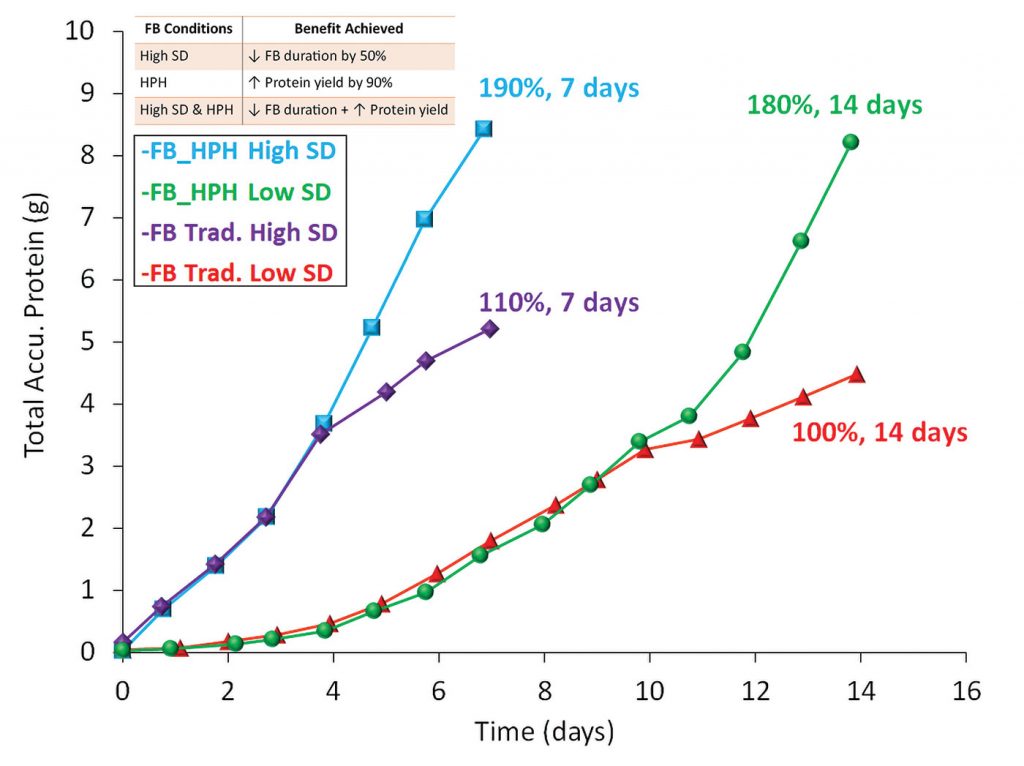
The case study focuses on utilizing a (N-1) bioreactor in a perfusion mode that enables inoculation of the production fed-batch bioreactor at 10E6 cells/mL, 20× higher compared to traditional seeding density. Increased seed density facilitates reducing the fed-batch culture duration by half. Implementing the HPH clarification process using the XCell ATF system resulted in an enriched environment with a 190% boost in the overall yield in half the fed-batch culture time.
The HPH process is performed as a single step in a sterile manner with a 0.2 µm clarified product as the output. The product is readily available for either batch or continuous chromatography without additional processing.
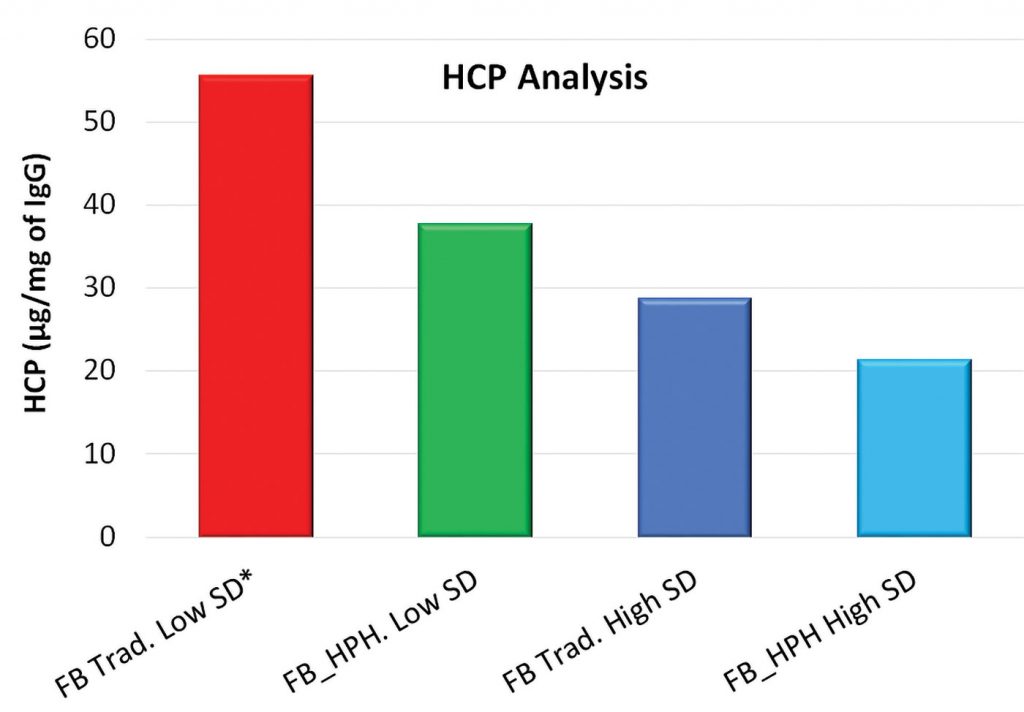
An additional benefit is the reduction in HCP and impurity production in the final bioreactor, further aiding downstream purification.
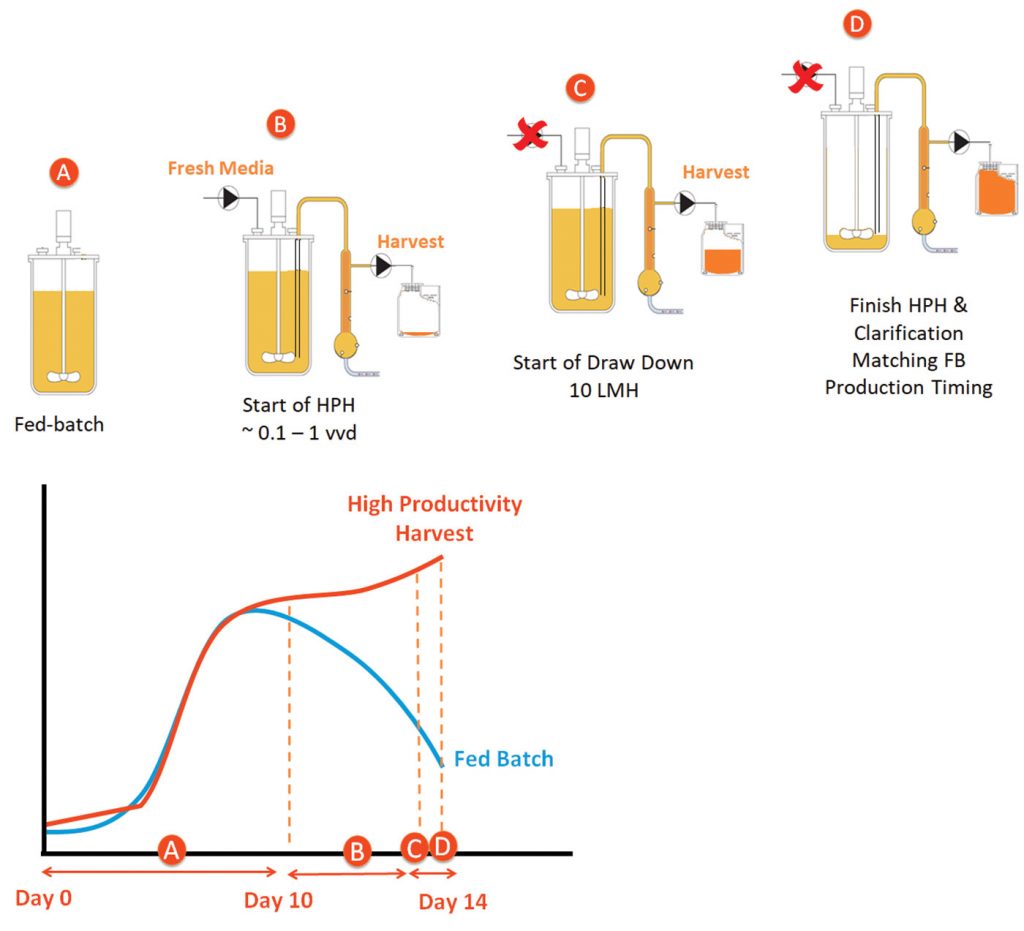
The fed-batch process is started per typical procedure (Step A) in Figure 2. Once the peak cell density is achieved, Day 10 in this example, fresh media is pumped in at a set flow rate (Step B). As fresh media is pumped in, product harvesting is initiated. On the last day of the run, media addition ends and draw down of the volume in the reactor begins at 10 LMH (Step C, ~4–8 hours). The HPH process is completed to match the original fed-batch production timing.
Materials and methods
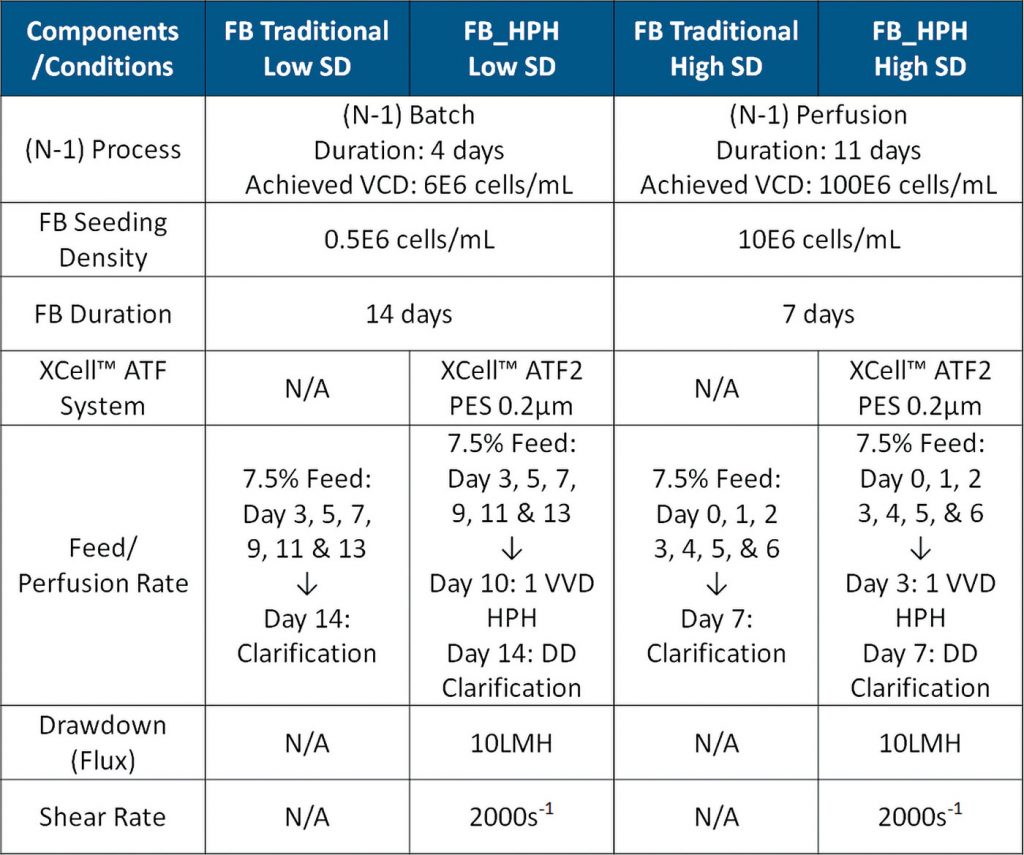
High-expressing mammalian CHO-GS was selected to evaluate the HPH process (Table). These cells were grown in Ex-CELL FB medium supplemented with 100 ng/mL LONG®R3 IGF-I, 4 g/L Poloxamer, and 4 mM Glutamax. This cell line is reported to express IgG, trastuzumab. All cultures were conducted using 3:L (w.v.: 1.2 L) Applikon glass bioreactors. The cell line and the method for a traditional fed-batch process (including feeding strategy) is provided by ATUM and Horizon Discovery.
Results
Enabling the (N-1) bioreactor with perfusion technology (Figure 3) achieved a VCD of 100E6 cells/mL in 11 days (data not shown). This intensified seeding process allowed the fed-batch cultures to be inoculated with 10E6 cells/mL (purple & blue)—20× higher seed density than FB traditional (green & red).

Inoculating with a higher SD shortened the culture duration by 50% for both the fed-batch traditional and HPH processes. Implementing HPH increased the viable cell density threefold (blue & green) compared to the fed-batch traditional cultures (purple & red).
Employing a high seed density culture (purple & blue) resulted in 10–15% higher protein productivity compared to low seed density fed-batch processes (red & green), as shown in Figure 4.
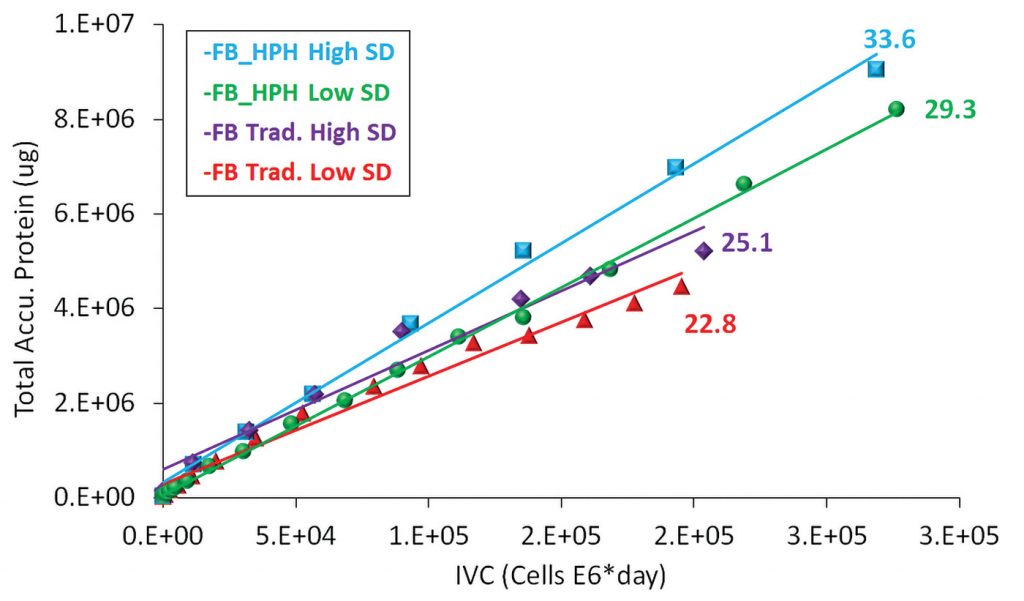
Implementing HPH increased the cell-specific protein productivity by 30% (blue & green). Sustaining high protein productivity translates to better product quality.
High seeding density shortens the production fed-batch culture duration and allows facilities to process more batches/year (Figure 5). Combining both applications (high seeding density and HPH) enables increased efficiency and productivity of an upstream facility.
A four-day HPH process at 1 vessel volume/day increases the total clarified volume 5× as compared to a traditional fed-batch process. Performing ultafiltration to concentrate the harvest material OR continuous capture chromatography can mitigate this concern.
Host cell protein values are lower for cultures where HPH is implemented (Figure 6). Cultures with high viability produced less host cell protein, indicating less process related impurities for further downstream applications.
Conclusions
A production bioreactor seeded with a high cell density and operated with the HPH process results in significantly increased product yield, improved process efficiency, and smaller operational footprint. The productivity and process benefits include:
- Up to 190% boost in protein production
- 50% reduction in production culture duration
- Elimination of centrifugation and depth filtration equipment and operations
- Healthier culture, which translates to lower HCP and lower process-related impurities
- Closed system and single-step process
- Harvested material that is 0.2 µm filtered and ready for purification
Shashi Kudugunti, PhD ([email protected]), is senior scientist, Daniel Diggins serves as senior research associate, Jyoti Amatya works as application scientist II, and Jamie Peyser is senior director at Repligen.


