Cell therapy developer Celyad Oncology is using short-hairpin RNA (shRNA) to create T cells that can be used as an “off-the-shelf” allogeneic therapy for cancer patients. They are also using the technology to help T cells survive longer in patients.
shRNA is an artificial RNA molecule with a tight turn that silences gene expression by neutralizing messenger RNA. According to David Gilham, PhD, CSO of Celyad Oncology, they are using this well-established technology to introduce shRNA alongside the therapeutic transgenes used in existing CAR-T therapies.
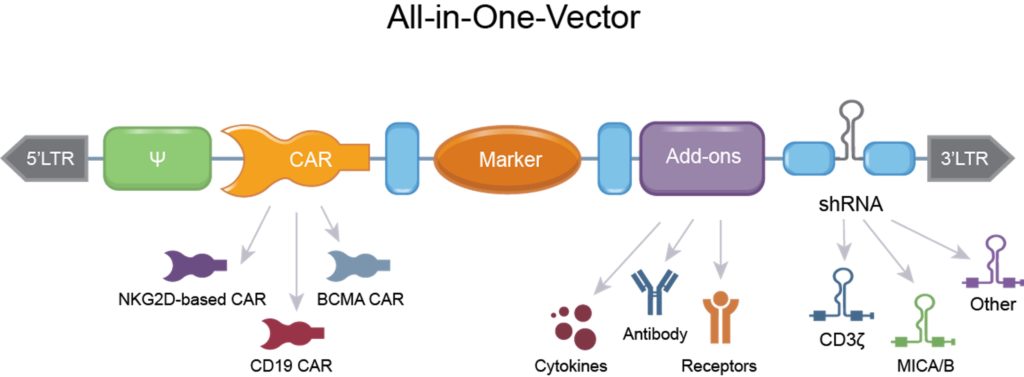
“We can introduce all the elements we want via shRNA in a single genetic modification,” he explains. “This keeps the cost of manufacturing, and therapies, as low as possible.”
The company uses a polymerase II promoter to express shRNA. This promoter also drives therapeutic transgenes, meaning shRNA can be added to the same line of genetic code as a transgene gene edit. In addition, according to Gilham, the amount of shRNA production is more controllable than with a traditional polymerase III promoter.
“Most people working on shRNA are using the older PolIII promoter system, so they have issues with stability of expression, overexpression, and toxicity,” he says. With PolII promoter, “the expression of shRNA is more controlled—that’s a key tweak.”
Using shRNA is also more straightforward than gene editing for manufacturing. According to Gilham, it doesn’t require multiple gene edits for modifying the activity of multiple genes. You can introduce multiple shRNAs in a single line of genetic code.
“We’ve got FDA approval for using just a single shRNA, but the exciting part to me is that, in the future, you can express multiple shRNA and can knockdown potentially any gene you’d like,” he says. In preclinical studies, the company have expressed up to four different shRNA from the same gene edit.
The company is currently in Phase I trials of an allogeneic CAR-T product, which uses shRNA to suppress the T-cell receptor (TCR) in T cells. They hope this TCR suppression will allow donor T cells to be used “off-the-shelf” in patients.
Also, in Phase I trials is an autologous CAR-T therapy. Here, patient T cells are modified with shRNA to reduce specific CAR T ligands expression from the cell surface, which should allow them to survive longer in the body.