As species continue to face massive extinction, preserving genetic material through judicious biobanking to enable cloning is key to promoting the survival of species and maintaining biodiversity.
Embryos and germ cells are commonly stored at ultra-low temperatures in liquid nitrogen. But storing reproductive cells in liquid nitrogen is tricky. Not only does it require considerable skill and maintenance costs, it also needs regular monitoring, and biomaterials stored in liquid nitrogen can be easily destroyed during power outages and other disasters. Therefore, it is crucial to develop methods where genetic material can be preserved long-term with minimal resources that enable the cloning of viable and fertile offspring upon prolonged storage.
Scientists at the University of Yamanashi in Kofu, Japan, have developed a new method that uses freeze-dried somatic cells—cells other than reproductive cells—to clone mice. The findings were published in an article in the journal Nature Communications titled, “Healthy cloned offspring derived from freeze-dried somatic cells” on July 5, 2022. Teruhiko Wakayama, PhD, a professor at the department of environmental sciences at the University of Yamanashi is the senior author of the study. The authors claim the new method could be used to store genetic material from any animal in a safe and inexpensive manner.
In an earlier study, Wakayama’s team had developed a freeze-drying technique for mouse sperm cells. Although the researchers could not retrieve healthy and functional sperm cells following the freeze-drying process, they could retrieve sperm DNA which they injected into oocytes to clone mice offspring. They later demonstrated that the technique worked in other species including rats, hamsters, rabbits, and horses. This increased the stability and security of storage.
The researchers observed that freeze-dried mouse sperms are highly resilient against fluctuations in the environment. Wakayama’s team successfully obtained healthy mice offspring from free-dried sperm stored in a desk drawer for over a year and at the International Space Station for more than 5 years. This convinced Wakayama and his team that freeze drying is the best and safest way to store genetic material for extended periods at low cost in any location.
However, until now, whole animals have only been cloned from freeze dried mature sperms. Collecting functional sperms, particularly from infertile males, as well as collecting female eggs from ovaries or fertilized embryos pose significant challenges for biobanking.
Since Wakayama reported cloning whole animals from freeze dried sperms DNA, frog and sheep have been successfully cloned from somatic cells, indicating that the storage of gametes is not essential as a genetic resource. Moreover, somatic cells can be easily collected from anywhere in the body, including body waste and following death.
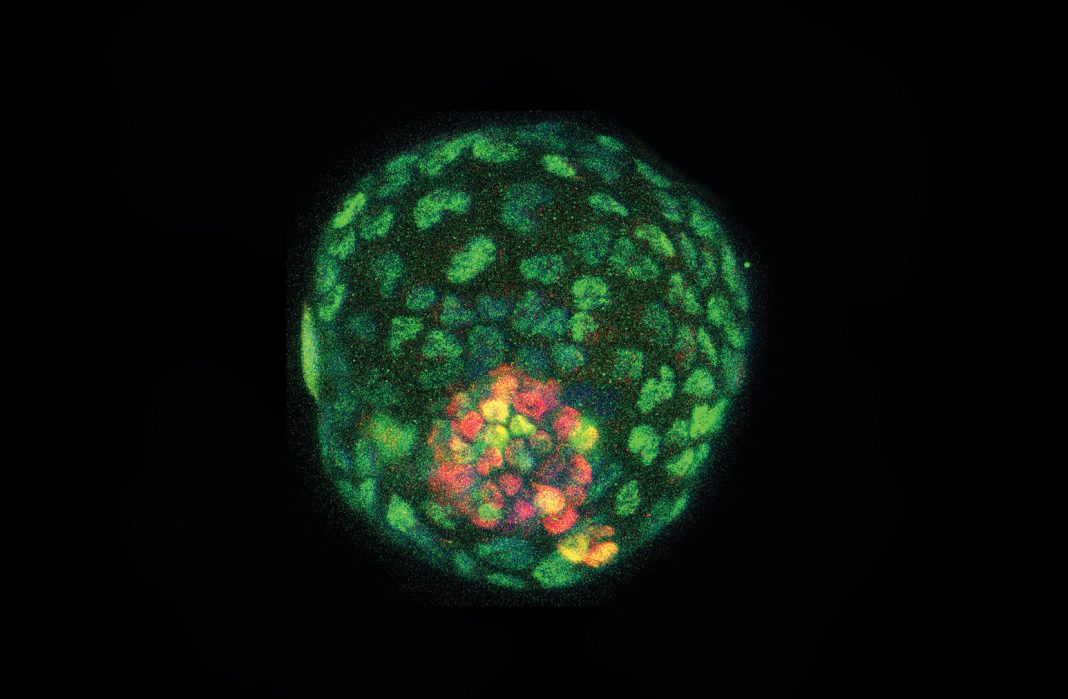
In the current study, Wakayama’s team generate healthy cloned mice offspring from freeze dried somatic cell nuclei through an adapted nuclear transfer procedure. They used fibroblast cells from the tip of mice tails. “Our data reveal that although some DNA abnormalities are observed in the process, freeze dried somatic cell nuclei can be used to generate blastocysts by nuclear transfer, and embryonic stem cell lines derived from these blastocysts yield donor nuclei that are capable of producing healthy, fertile cloned mice,” the authors noted.
The researchers freeze-dried the somatic cells for up to nine months at -30°C, using trehalose as a cryoprotectant or epigallocatechin as an antioxidant. When they attempted to revive the cells with rehydration and stained them with propidium iodide, they observed that the cell membranes were damaged, and the cells were dead.
Nevertheless, the authors used the DNA from these cells as donor material for cloning to generate healthy female and male offspring, with a success rate of 0.2–5.4%. They adapted the procedure for somatic cell nuclear transfer to generate embryos (blastocysts) and stable embryonic stem cell lines.
“After nuclear transfer, we produced cloned blastocysts from freeze-dried somatic cells, and established nuclear transfer embryonic stem cell lines,” the authors noted.
In addition, the investigators selected nine female and three male cloned mice and allowed them to mate. All females delivered litters, indicating fertility is retained in the cloned animals.
Comet assays on the freeze-dried cells revealed that the DNA in these cells underwent more damage than observed following storage in liquid nitrogen. However, the authors contend, the cloning success rate achieved indicates the new method may provide a viable alternative despite the DNA damage, since it provides a cost-effective and long-term solution.
“We show that freeze-dried somatic cells can produce healthy, fertile clones, suggesting that this technique may be important for the establishment of alternative, cheaper, and safer liquid nitrogen-free biobanking solutions,” the authors concluded.