Demeetra AgBio reports that it is advancing its pipeline for therapeutic cannabinoids and expects to file an IND for its lead candidate with the FDA early next year.
The company’s Cas-CLOVER™ gene editing tool, in combination with its bioprocessing capabilities, will allow it to discover and manufacture rare and novel cannabinoids, creating opportunities for clinical and consumer applications, according to CEO Jack Crawford, who notes that Demeetra is seeking gene editing collaborations with cannabis genomics and cell culture experts.
Demeetra also is developing processes to manufacture biotherapeutics at scale, using yeast and CHO cell lines as bioprocess platforms. Demeetra has partnered with big pharma and contract manufacturers in the biotherapeutics space.
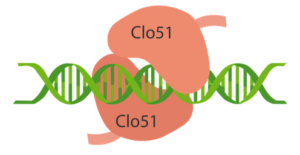
As opposed to the use of a single guide RNA (gRNA) for sequence-specific gene editing, the Cas-CLOVER system utilizes a dual gRNA in addition to the Clo51 nuclease activity that requires dimerization of subunits associated with each guide RNA, according to a company spokesperson, who says this allows for more stringent DNA cleavage. Using two gRNA makes the Cas-CLOVER gene editing system highly restricted and only functional when the paired gRNAs coexist, adds the Demeetra official, who points out that the gene editing system has been validated in CHO cell lines and yeast for bioprocessing, as well as plants for crop trait engineering.
Headquartered in Kentucky, Demeetra AgBio was launched in 2019 as a spin out of Transposagen Biopharmaceuticals.


