Sponsored content brought to you by
In the gene and cell therapy field, there is a clear need to have cost-effective, scalable processes in the development of successful therapies utilizing viral vectors. Use of ultracentrifugation (UCF) has long been the desired process for viral vector purification and even considered by many to be the gold standard for purification. However, there is also the belief that while UCF achieves high levels of purification, the process is not scalable. Data contained herein demonstrate that continuous flow UCF is linear and scalable.
One of the primary goals of this work was to not only demonstrate linear scalability but to also identify key parameters in the process. Iodixanol was selected as the desired gradient for this experiment because of its common use in AAV purification.
In these experiments iodixanol was used across three ultracentrifuge systems where only volumetric loads and production flow rates changed. The instruments utilized to generate this data are the Alfa Wassermann Promatix 1000™, PKII, and KII ultracentrifuge systems. These systems are representative of development scale to production scale volumes. Analysis included not only manual fluid control but also automated fluid management by utilizing Alfa Wassermann’s Automated Fluid Handling (AFH) system. Critical factors identified to achieve scalability included consistent rotor core minimum and maximum radius along with maintaining gradient density. Across the three instruments the variable factor was fluid volume. When these criteria are utilized linear scalability is achieved.
Materials and Methodology
Suitable volumes of 25% and 50% (w.v.) Iodixanol solutions in water were produced and densities confirmed using refractometers. The process was set up in manual and fully automated modes to include all steps of separation, priming, de-bubble, gradient loading, product loading, and fraction collection per the rotor volume.
The graphs below demonstrate that the data generated for each run showed similar peak height and width and the figures produced similar gradient shapes across the Alfa Wassermann range of ultracentrifuges. The gradient remains identical throughout the volumetric differences between each separation. Product separation at the iso-dense layer and equivalent product peak shape in the gradient for each scale rotor assembly were achieved. Scalability and linearity of the particle separations are achieved. Scalability is demonstrated because the run parameters remained the same, even though rotor assembly volume varied.
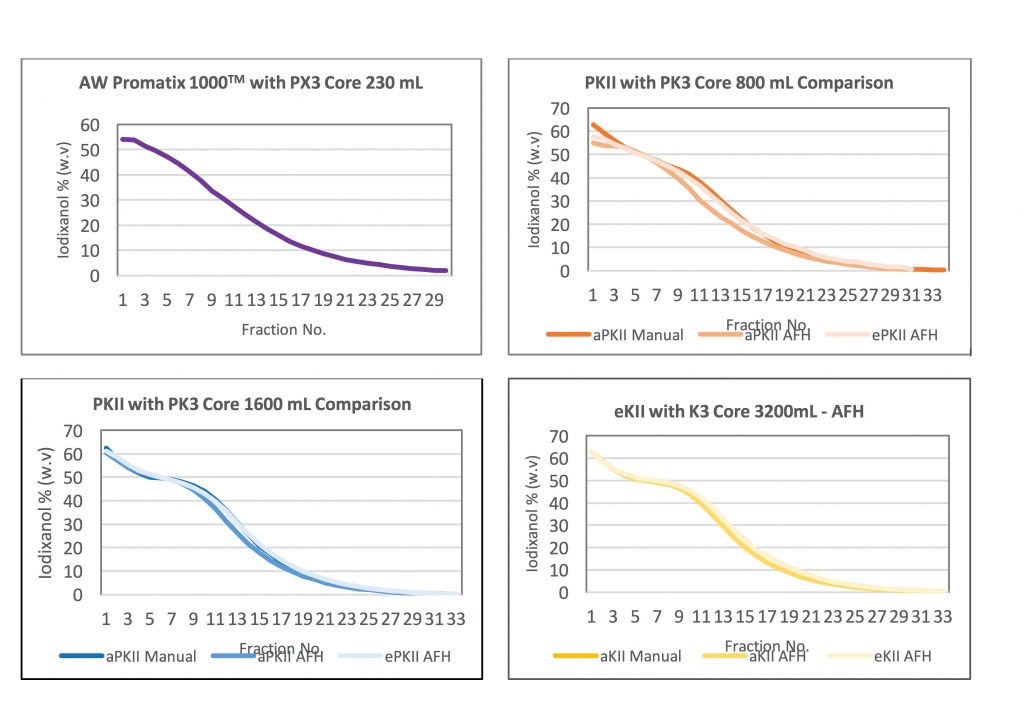 In summary, when gradient density and rotor minimum/maximum radius are consistent, product production from research and development to full scale production volumes are achievable. A solution to cost effective, scalable gene and cell therapy product development is found in continuous flow ultracentrifugation.
In summary, when gradient density and rotor minimum/maximum radius are consistent, product production from research and development to full scale production volumes are achievable. A solution to cost effective, scalable gene and cell therapy product development is found in continuous flow ultracentrifugation.
Alfa Wassermann Separation Technologies (AWST), a subsidiary of Alfa Wassermann Inc., is a supplier who, for over 60 years has had the singular purpose to support the bioprocess and pharmaceutical industries by providing expertise in continuous flow ultracentrifugation separation applications, fluid management solutions, and process monitoring.
AWST’s continuous flow ultracentrifuges efficiently and reliably separate viruses, virus like particles, and viral vectors for the development and manufacture of vaccines, gene therapies, and other bio-products. With the addition of the AWST Automated Fluid Handling (AFH), customers now have a system that fully automates and standardizes critical bio-manufacturing processes.
For more information visit: www.AWST.com


