Sponsored content brought to you by
In early December 2021, the FDA granted emergency use authorization (EUA) to AstraZeneca for a long-acting antibody combination that protects against COVID-19. A team of scientists led by James Crowe, MD, and Robert Carnahan, PhD, at the Vanderbilt Vaccine Center discovered the original highly potent antiviral antibodies that were the basis for the engineered long-acting antibodies.

Associate Director of the Vanderbilt Vaccine Center, Associate Professor in Pediatrics and Radiology
The monoclonal antibody (mAb) combination, called Evusheld, was authorized as a pre-exposure prophylaxis to prevent COVID-19 in adults and children 12 years and older who have compromised immune systems or a history of severe adverse reactions to a COVID-19 vaccine. “The emergency use approval of these antibodies for pre-exposure prophylaxis is a game changer for millions of vulnerable and immunocompromised individuals. We could not be more excited to see these move into clinical use,” said Carnahan, who is Associate Director of the Vanderbilt Vaccine Center.
The Race to Respond to a Pandemic
Although antiviral human mAbs are promising drug candidates for preventing or treating severe viral diseases, typically the long timelines for discovery, functional analysis, preclinical studies, and manufacturing can limit their rapid deployment.
The U.S. Defense Advanced Research Projects Agency (DARPA) is working to change this paradigm. Launched in 2017, their Pandemic Prevention Platform (P3) focuses on rapid discovery, characterization, production, testing, and delivery of efficacious DNA- and RNA-encoded medical countermeasures against infectious disease. The Vanderbilt Vaccine Center is one of four DARPA performer sites.
In 2019, Vanderbilt Vaccine Center’s first DARPA 90-day challenge targeted the Zika virus,1 with Crowe’s laboratory focused on antibody discovery. This collaborative effort involved scientists from the Ragon Institute of Massachusetts General Hospital, MIT, Harvard, Infectious Disease Research Institute (IDRI), Washington University, and Beth Israel Deaconess Medical Center.
“We accomplished this challenge in 78 days. In our second challenge, we wanted to reduce the time to 60 days and planned to focus on a novel influenza virus,” said Carnahan. “Then SARS-CoV-2 began to escalate to pandemic scale, and we segued. In late January 2020 we received a sample from the first U.S. infected individual.”
Identifying Highly Potent Neutralizing Antibodies with an Innovative Functional Assay
The day the sample was received, peripheral blood mononuclear cells were enriched for antigen-specific memory B cells. “After the survey of antibody-secreting B cells and single-cell functional analysis, we performed rapid functional screening for potent neutralizing mAbs,” said Pavlo Gilchuk, PhD, Senior Staff Scientist, Vanderbilt Vaccine Center.
The team had two goals: first, to screen the large mAb panel for neutralizing activity against SARS-CoV-2 and then to rank them by their potency. “We used the 384-well Agilent xCELLigence RTCA HT platform to perform an objective, automated screen that provided high-throughput, rapid, quantitative, and real-time cytopathic effect (CPE) monitoring,” said Gilchuk. “The principle of detection was cell impedance, expressed as a cell index that declines as CPE increases. The measurements provided real-time neutralization activity for each antibody.”
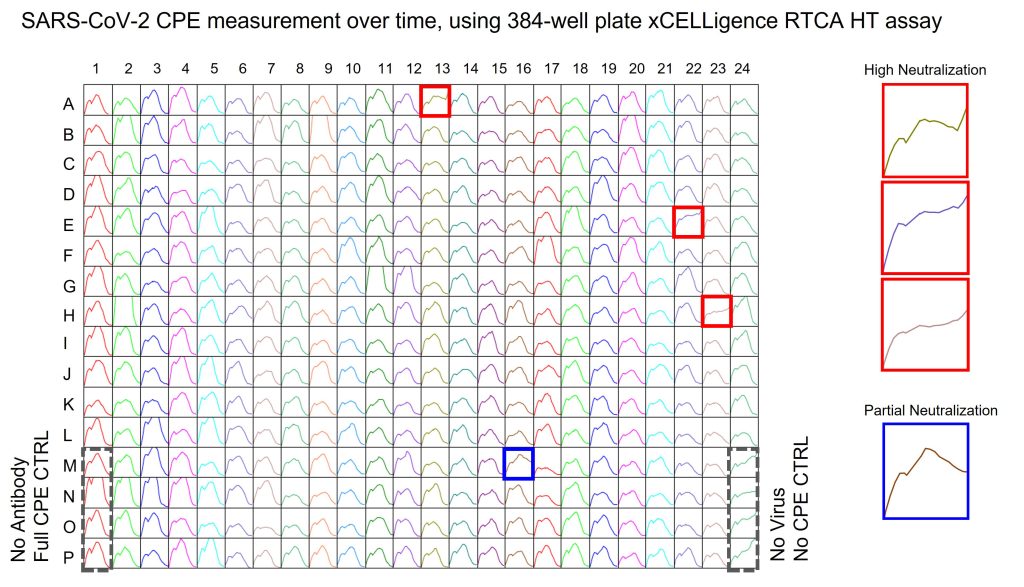
In addition, the neutralizing activity measured by xCELLigence RTCA does not require custom labels or reagents, enabling the team to quickly adapt the antibody workflow developed for the Zika challenge to the novel coronavirus. “We were able to bypass a multitude of hands-on, multistep processes required by plaque or focus-forming assays to measure viral activity, such as wash, fix, and stain steps, and avoided having to develop custom virus-specific antibodies for staining,” said Carnahan.
Using xCELLigence RTCA, the team identified 70 neutralizing antibodies against SARS-COV-2, most of which mapped to the RBD (receptor binding domain), identifying it as a potential site of vulnerability. “To rank neutralizing antibodies by their potency, each mAb was tested at four dilutions in the same screening study,” said Gilchuk. “This allowed us to estimate IC50 values.” In contrast, traditional antibody-binding assays result in a random assortment of antibodies, from low to high function. “Every effort should be made to down-select from the beginning on function. We have seen often that binding is required for function, but they do not correlate in magnitude. Since highly functional antibodies are rare, the project would have been at a major risk of not getting the most potent antibodies without a functional neutralizing assay,” said Carnahan.
Follow-on orthogonal focus-forming and eGFP-reporter neutralization assays confirmed the activity of the lead neutralizers. Animal studies also showed results that correlated well with the RTCA data, further indicating that RTCA was able to identify important neutralizers of SARS-CoV-2 from a large panel of recombinant mAbs.
This work was published in Nature Medicine2 in 2020.
The Road to Approval
During the DARPA sprint, the team also kept an eye on other needs. This included forging relationships with companies to make the mAbs available to researchers.
“Some of the antibodies have been used for pathology studies, and a number of companies are working to deploy antibody-based screening platforms that incorporate some of our antibodies,” said Carnahan. “In addition, these mAbs can be used to support vaccine development. They are a great tool for lot release and clinical studies. Several of our antibodies are being used by vaccine manufacturers, as part of their development.”
In June 2020, six of the monoclonal antibodies were licensed to AstraZeneca for optimization and advancement into clinical development. A combination of two long-acting antibodies were advanced into Phase III clinical trials in January 2021, and emergency use authorization (EUA) was granted by the FDA by December 2021.
Evusheld recipients had a 77% reduced risk of developing COVID-19, which was maintained through six months. Additionally, studies from University College, Oxford, U.K. and Washington University School of Medicine, St. Louis, U.S. have found that the long-acting antibody combination retains neutralization activity against the Omicron SARS-CoV-2 variant. “These will also be the first COVID-19 targeted antibodies to be administered through standard injection rather than an infusion process, further reducing the barriers to access,” said Carnahan. “Effective and accessible antibody therapies like these are likely to grow in utility and become a key tool in our infectious disease toolbox.”
About Vanderbilt University Medical Center—Crowe Lab
The James Crowe laboratory has a broad portfolio of work in the area of viral immunology and antibody sciences, with an aim to discover mechanisms important to develop new therapeutics and vaccines. They use a very broad array of techniques including molecular and cellular biology, state-of-the-art imaging and flow cytometry, bioinformatics, and bioengineering approaches to attack the scientific problems.
About Agilent xCELLigence RTCA Instruments
The Agilent xCELLigence Real-Time Cell Analysis (RTCA) instruments use label-free cellular impedance to continuously monitor cell behavior with high sensitivity and reproducibility. The RTCA virus-neutralizing antibody assay is a highly efficient replacement for the plaque reduction neutralization test (PRNT). Simply plate host cells, then add virus in the presence of neutralizing antibody. No further hands-on time is required. Data is acquired continuously without the use of agar, and neutralizing activity is detectable in as little as one hour. Learn more at www.agilent.com/chem/CPE.
References
1. Gilchuk, Pavlo et al. “Integrated pipeline for the accelerated discovery of
antiviral antibody therapeutics.” Nat. Biomed. Eng. 4, 1030–1043 (2020).
2. Zost, S.J. et al. “Rapid isolation and profiling of a diverse panel of human
monoclonal antibodies targeting the SARS-CoV-2 spike protein.” Nat. Med. 26,
1422–1427 (2020).