Sponsored content brought to you by

Success in gene and cell therapy is driving demand for plasmid DNA which is a key reagent to produce viral vectors, both for commercial products as well as the breadth of different vectors for the expanding number of programs in development. Standardization and large-scale production of helper and packaging plasmids, those that are the same regardless of the specific viral vector produced, significantly reduce timelines, costs, and risks.
To address this growing need, Aldevron has developed and deployed a production program around a single-use 300L fermentor and process train capable of purifying up to 100 grams of a plasmid in a single processing event in as little as seven days. Data generated proved that fermentation scalability supports large processing events.
Scaling work done in the early engineering phase for this train was integral to the design of a new 70,000 square foot Fargo-based manufacturing plant for plasmid DNA production. New 1,000 L fermentation capacity and a planned expansion of the company’s Madison-based manufacturing footprint by nearly 10,000 square feet will augment capacity.
“Our results demonstrate process scalability and show that standardized plasmids can be used for viral vector production as an attractive alternative to custom manufacturing, which can take months. Given the stability of plasmid DNA, standardization allows us to maintain an inventory of these products for clinical and commercial production of viral vectors. The immediate “on demand” availability, lower cost, and freedom to operate enable a faster and more economical path for gene therapy product development,” said James Brown, VP Corporate Development, Aldevron.
Standardization enables plasmid inventory available “on demand”
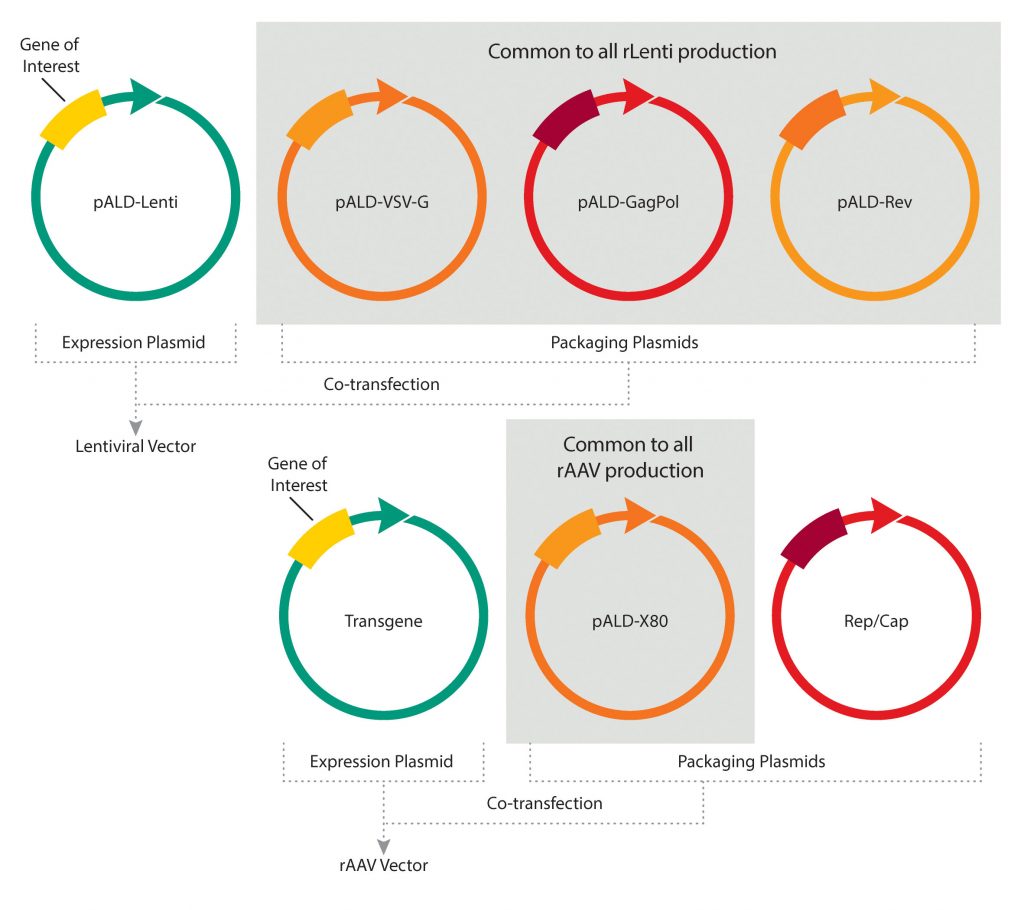
Several methods have been developed for producing recombinant adeno-associated virus (rAAV) and recombinant lentivirus (rLenti), the most widely used viral vectors. One of the most common is transfection of plasmid DNA into HEK293 or HEK293T cells. This method requires between two and four separate plasmids, the most critical production components, depending on the vector and arrangement of genes in the plasmids.
Triple transfection of three plasmids into HEK293 cells is the most frequently used rAAV production method. The helper plasmid in this scenario is independent of the transgene or AAV serotype. A similar production method for rLenti uses four plasmids with three packaging plasmids also independent of the transgene.
Consistency from development through commercialization
For rAAV production the pXX6-80 plasmid published in 1998 by Xaio, et al. (J Virol 72: 2224–32) is an attractive choice given its wide use to produce rAAV for discovery research and clinical trials. A version of this plasmid with the same genes and structure, named pALD-X80, is commercially available and includes kanamycin resistance, which is preferred by regulatory agencies over ampicillin.
Aldevron data comparing transduction of rAAV produced with pALD-X80 and another commercially available rAAV helper plasmid using two different plasmid production methods are generally consistent with the large body of published literature supporting the performance of pXX6-80 for rAAV production.
The four-plasmid system of rLenti production represents an even greater opportunity to impact costs and timelines with standardized plasmids. Developed by Oxford Genetics the pALD Lenti plasmids are optimized for rLenti production. The pALD-Rev and pALD-VSV-G plasmids have been codon optimized and extraneous sequences minimized. HIV/VSV and inter-cassette homology have also been minimized.
Large-scale batches of the rAAV helper plasmid, pALD-X80, are now available from Aldevron for immediate delivery at research and GMP-Source clinical quality grades. Large-scale batches of lentiviral plasmids, pALD-Rev, pALD-VSV-G and pALD-GagPol are also available at research grade; GMP-Source clinical grade quality will be available mid-2019.
No royalties, milestones, or other reach-through costs
In addition to cost and supply chain complexity, innovators in genetic medicine must also be concerned with intellectual property if they desire to bring therapies to patients as quickly as possible. The pALD-X80 and pALD-Lenti plasmids do not have royalties, milestones, or other reach-through costs. The combined features of these plasmids make them excellent options for gene therapy development and commercialization.

