Sponsored content brought to you by
One of the key challenges of developing antibody therapeutics is finding antibodies with the desired function. Many of the “low-hanging fruit” therapeutics have already been identified using traditional technologies like hybridoma and phage display. As the market now turns to more difficult targets, such as antibodies that elicit weak immune reactions or targets with rare epitopes, it has become clear that progress is severely hampered by a lack of technologies that allow access to greater diversity and enable functional screening of the entire B-cell repertoire, which is inaccessible by traditional techniques.
Plasma B cell cloning using fluorescence-activated cell sorting (FACS) has emerged as a powerful tool for increasing accessible antibody diversity because it allows enrichment of highly diverse plasma B cells based on the expression of known B cell markers. However, FACS does not enable functional screening of the antibodies produced by these B cells, and hence these screens can neither identify antigen-specific hits nor down select hits to identify promising lead candidates for lead optimization and preclinical development.
Plasma B cell–based antibody discovery on the Beacon® platform changes this by enabling automated, direct functional screening of B cells immediately after organ harvest and cell purification. B cells can be screened from multiple organs (spleen, bone marrow, lymph nodes) and cultured for multiple days in specialized media to enable multiple screens of a single plasma B cell sample to access greater B cell diversity.
The Beacon enables discovery of thousands of hits in a single workflow and down selection of lead candidates using multiple assays for antigen specificity and function. Tens of thousands of plasma B cells are gently guided into nanoliter-sized NanoPen™ chambers on a microfluidic chip using light (Figure 1). The small size of these chambers enables rapid, precise, and highly sensitive assays that can be completed in less than 1 hour. Chips can then be reset to enable assays for deeper interrogation of the same antibodies. This thorough upfront characterization reduces the expense of sequencing or cloning irrelevant nonfunctional hits.
Following functional characterization, OptoSeq BCR enables on-chip genomics integration to enable simple and efficient recovery of paired heavy/light chain sequences. Plasma B cells are lysed in their respective NanoPen chambers, and mRNA is captured on beads for on-chip cDNA synthesis. Sequences associated with desired function can then be recovered for several days without the sequence degradation that occurs with the death of fragile plasma B cells. Using this approach, we were able to recover >650 unique antigen-specific, functionally characterized heavy/light chain antibody sequences from a single automated workflow in less than one week (Figure 1).
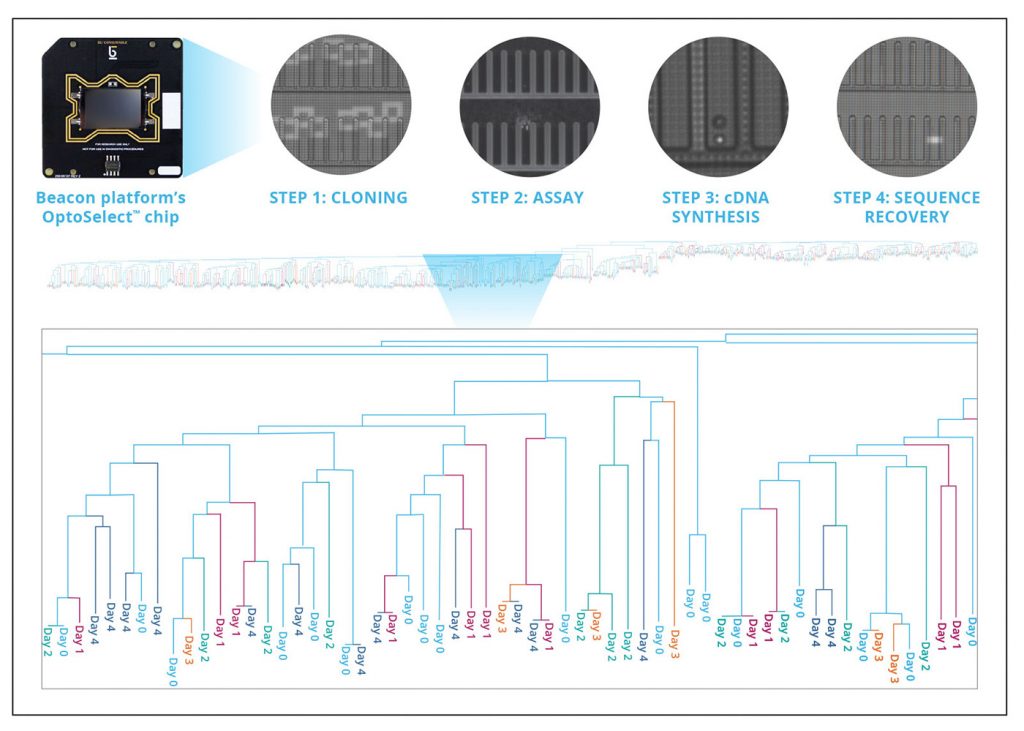
Access to this level of functionally characterized diversity in such a short timeframe alleviates a key bottleneck in the development of antibody therapeutics and finally provides ready access to the elusive “high-hanging fruit” —antibodies against difficult targets.
To learn more and see the full data set, visit berkeleylights.com/DiversityPosterGEN


