*CEVEC Pharmaceuticals, Cologne, Germany;
#FH Aachen University of Applied Sciences, Aachen, Germany
Sponsored content brought to you by

Viral vectors are gaining increasing importance in various medical applications. Among the different types of viral vectors, adenoviral (Ad) vectors have been used for a long time and have now received renewed attention due to the recent approvals of Ad vector–based COVID-19 vaccines Vaxzevria (Oxford/AstraZeneca) and Janssen COVID-19 Vaccine (Johnson & Johnson). In addition to vaccine production, Ad vectors are also used as one of the most suitable delivery tools in gene therapy applications and as oncolytic viruses.
Among the advantages of Ad vectors for vaccine production is their favorable safety profile, the ability to stimulate a robust cellular and humoral immune response, the efficient transduction of both, proliferating and quiescent cells, the relative ease of vector construction, the possibility of rapid virus production with high titers, and their stability (which facilitates purification and long-term storage).
A crucial safety feature of Ad vectors as delivery vehicles in vaccine production or gene therapy is their replication incompetence. This means that the virus, having introduced its cargo into the patient’s cells, cannot multiply in an uncontrolled manner. To achieve this, the Ad wild-type genome was modified in a variety of ways. The Ad∆E1 vectors most commonly used in vaccine production and gene therapy carry a deletion of the E1 region (400-3500), which is indispensable for virus replication. In addition, the adenoviral E3 gene, which is not required for replication, is usually deleted in these vectors, creating further space for the insertion of the transgene.
Due to the E1 deficiency in Ad∆E1 vectors, special producer cells that trans-complement the E1 function are required to produce replication-incompetent Ad viruses (Figure 1A). Several cell lines for manufacturing such vectors have been described, of which HEK293 is the most common. The HEK293 cells are derived from human embryonic kidney cells which were immortalized by sheared Ad5 virus DNA.1 They contain a single insertion of bp 1-4344 from the Ad5 genome. Accordingly, a significant homology remains between Ad∆E1 vectors and the Ad5 sequences inserted into HEK293 cells. This homology allows for homologous recombination events to happen during the production process, leading to the risk of generating replication-competent adenovirus (RCA) (Figure 1B, left).2 The presence of RCAs in clinical batches is highly undesirable, as the replicating virus can trigger pathogenic processes and a strong immune response.3–4 Accordingly, the FDA currently sets a limit of less than 1 RCA in 3 × 1010 viral particles in adenoviral vector preparations. As preparations must be tested for RCA and discarded if they exceed the allowed threshold, this can lead to serious cost and timing issues in vector manufacturing.
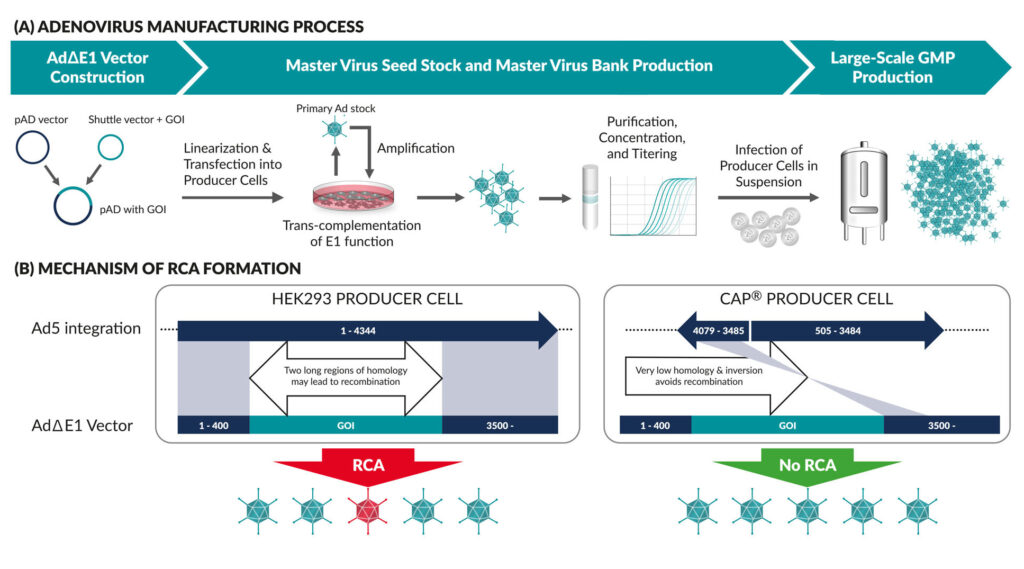
An immediate solution for RCA-free production is offered by CAP® cells, a derivative of the cells originally described as N52.E6,5 specifically developed for GMP manufacturing. Human amniocytes were isolated during a routine amniocentesis and subsequently immortalized with AdE1A/E1B and pIX gene functions. The latter was attributed an important function in stabilizing the Ad capsid and providing temperature resistance, both being important for commercial manufacturing.6 The special arrangement of elements in the plasmid used to immortalize CAP® cells reduces the risk of RCA occurrence dramatically (Figure 1B, right). Indeed, numerous studies have demonstrated the absence of these contaminants in CAP®-produced Ad vectors. Other features of CAP® cells include their nontumor and ethically acceptable origin; their single cell suspension growth to high cell densities in serum-free, chemically defined media; and their compatibility with all common bioreactors and easy scale-up to high volumes. Most importantly, CAP® cells support high-titer Ad vector production. The availability of GMP-compliant materials and protocols and an FDA-deposited Biological Master File complement the suitability of CAP® cells as a platform for RCA-free, large-scale Ad vector production.
References
1. Graham, F.L. et al., J Gen Virol. 36(1) (1977)
2. Murakami, P. et al., Hum Gene Ther. 13(8) (2002)
3. Lochmüller, H. et al., Hum Gene Ther. 5(12) (1994)
4. Hermens, W.T., Verhaagen, J. Prog Neurobiol. 55(4) (1998)
5. Schiedner, G et al Hum Gene Ther. 11(15) (2000)
6. Kovesdi, I., Hedley, S.J. et al., Viruses 2(8) (2010
Learn more cevec.com

