Sponsored content brought to you by

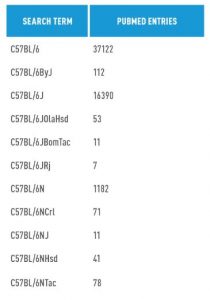
Certainly, substrain differences exist in all inbred mouse strains. By far, however, the C57BL/6 strains are the most commonly published in the world with over 37,000 entries in PubMed (Table 3). For this reason, this paper will focus on published differences only in the C57BL/6 family.
Currently there are over 16,000 entries using the original Jackson Laboratory C57BL/6J substrain. A few other entries exist for substrains derived from the original C57BL/6J. Roughly 1200 entries use C57BL/6N derived substrains. In the coming years, the use of C57BL/6N substrains is expected to grow significantly, as all 20,000 genes in the mouse genome will eventually be targeted in C57BL/6N ES cells through the International Knockout Mouse Consortium (IKMC) project (www.mousephenotype.org).
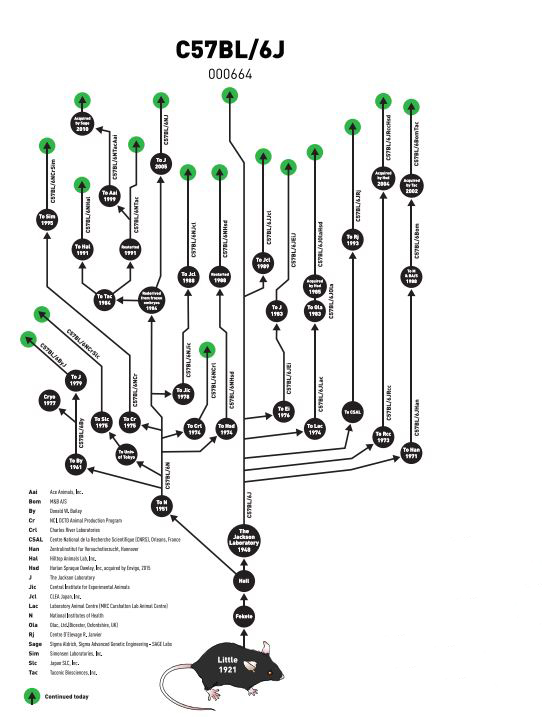
The original C57BL/6J substrain from The Jackson Laboratory was sent to the National Institutes of Health (NIH) in 1951. The NIH substrain, C57BL/6N, was later distributed to several institutes including Charles River Laboratories in 1974 (C57BL/6NCrl), to Harlan (now Envigo, C57BL/6NHsd) in 1974 and 1988, and to Taconic (C57BL/6NTac) in 1991. In 2005, the N substrain came back to The Jackson Laboratory, and is known as the C57BL/6NJ substrain. Currently, at least 100 generations of breeding separate C57BL/6J substrains from all C57BL/6N substrains (Figure 3).
Several publications demonstrate heritable phenotypic differences between J and N substrains that have arisen due to genetic drift. Depending on the specific research question, some substrains may be preferred over others (Bryant, 2011). Some classic and recent examples are listed here:
- C57BL/6J mice express a mutant Nnt gene, which is involved in glucose-mediated insulin secretion, compared to C57BL/6N substrains (Freeman et , 2006).
- C57BL/6J mice have strong preferences for alcohol while C57BL/6NCrl mice do not (Mulligan et , 2008). Quantitative Trait Loci mapping studies comparing these substrains may lead to a better understanding of the genes involved in addiction.
- C57BL/6N substrains harbor the retinal degeneration allele Crbrd8 while the C57BL/6J substrain carries a wildtype allele (Mattapallil et al., 2012).
- C57BL/6JOlaHsd mice are homozygous for a spontaneous deletion in the genes encoding alpha synuclein and multimerin-1 (Specht and Schoepfer, 2001, 2004). While, alpha synuclein aggregates in the nervous system in Parkinson’s Disease, the deletion in C57BL/6JOlaHsd substrain does not appear to contribute to prion disease mediated synaptotoxicity (Asuni et al., 2010) but may have effects on motor neuron degeneration in general (Pelkonen and Yavich, 2011; Peña-Oliver et al., 2012). C57BL/6JOlaHsd mice also have reduced bone density compared to C57BL/6J and C57BL/6JRccHsd substrains (Liron et , 2017).
- C57BL/6NHsd mice carry a Dock2 mutation affecting B cell signaling and immune tolerance that is not found in other major C57BL/6 substrains (Mahajan et al., 2016).
In this last recent example, an approximate 10 year step back in research progress for one laboratory occurred as a result of conclusions drawn from using 2 distinct C57BL/6 substrains over the course of their studies. (www.jax.org/news-and-insights/jax-blog/2016/may/why-it-took-2-years-for-a-harvard-research-lab-to-get-back-to-research). The original studies were published using an undefined “C57BL/6” substrain as the genetic background for creating a Siae gene knockout (Cariappa et al., 2009). Siae was thought to contribute to B cell development and signaling when initially published in 2009. The Siae mutation was later backcrossed to the specific C57BL/6J substrain from The Jackson Laboratory. Surprisingly, the experiments on the C57BL/6J background failed to reproduce the laboratory’s previous publication (Mahajan et al., 2016). After several years of additional analysis of several commercially available C57BL/6 substrains, it was discovered that a copy number mutation in a different gene, Dock2, had spontaneously arisen in a strain of C57BL/6NHsd mice. Dock2 was the actual causal mutation for these B cell functions. This example should serve as a cautionary tale to closely monitor and understand the origins of any mice used in research. Because of genetic drift, inbred mouse substrains should not be used interchangeably.
It should be noted that in addition to the specific research question, the phenotypic effects of spontaneous mutations that have arisen due to genetic drift may depend on several contributing experimental factors. For instance, the Nnt mutation in C57BL/6J strains has been shown to have reduced insulin secretion in vitro compared to C57BL/6J mice rescued with transgenic wildtype Nnt (Freeman et al., 2006). In another study, no significant differences in insulin secretion were measured in vitro or in vivo in C57BL/6J and C57BL/6NTac substrains (Wong et al., 2010).
Furthermore, the Nnt mutational status and relationship with diet-induced obesity and insulin responsivity is not straightforward, as it may depend on the fat content of the diet (Nicholson et al., 2010). Similarly, two J substrains (J, JWehi) and four N susbstrains (NTa NHsd, NCrl, NJ) fed a low fat diet were found to have similar insulin secretion profiles in response to glucose challenge. However, when fed a high fat diet, the C57BL/6NJ substrain demonstrated a reduced insulin response to glucose challenge that could not be explained by differences in Nnt status, weight gain, fat mass, food intake, or beta cell area (Hull et al., 2017).
Several other published differences exist between C57BL/6 substrains. Differences in behavior such as fear, anxiety, pain, and response to amphetamines have been noted in the literature (summarized in Bryant et al., 2008). More broadly, differences exist across many other baseline measures. In particular, C57BL/6J and C57BL/6NTac substrains were compared in a comprehensive, standardized phenotyping pipeline of 413 parameters (EMPReSS) completed by four individual mouse centers of the European Mouse Disease Clinic (EUMODIC) consortium (Simon et al., 2013). Across the four phenotyping centers, the J and NTac mice differed in several areas including startle response, locomotor activity, grip strength, cardiovascular characteristics, metabolic parameters, and clinical chemistries.
NHsd, NCrl, NJ) fed a low fat diet were found to have similar insulin secretion profiles in response to glucose challenge. However, when fed a high fat diet, the C57BL/6NJ substrain demonstrated a reduced insulin response to glucose challenge that could not be explained by differences in Nnt status, weight gain, fat mass, food intake, or beta cell area (Hull et al., 2017).
Several other published differences exist between C57BL/6 substrains. Differences in behavior such as fear, anxiety, pain, and response to amphetamines have been noted in the literature (summarized in Bryant et al., 2008). More broadly, differences exist across many other baseline measures. In particular, C57BL/6J and C57BL/6NTac substrains were compared in a comprehensive, standardized phenotyping pipeline of 413 parameters (EMPReSS) completed by four individual mouse centers of the European Mouse Disease Clinic (EUMODIC) consortium (Simon et al., 2013). Across the four phenotyping centers, the J and NTac mice differed in several areas including startle response, locomotor activity, grip strength, cardiovascular characteristics, metabolic parameters, and clinical chemistries.
Taken together, genetic background is one component of experimental design which may affect reproducibility and the ability to make generalizations about biological processes. Troublingly, of the nearly 37,000 entries in PubMed for “C57BL/6,” the majority of these publications do not indicate a substrain.