Sponsored content brought to you by
Considerable advancement in technology used in gene manipulation, editing, and gene delivery has led to a rapid development of viable therapeutics based on gene therapy strategies. As of 2022, the FDA has approved eight gene therapy products for clinical use.1 Candidates for gene therapy include cancers, hematological conditions, ocular diseases, neuromuscular disease, immunodeficiencies, and (frequently) rare or inherited disorders.
Key to the success of a gene therapy strategy is the development of an efficient process or method to deliver the gene product to the intended cell, tissue, or organ. Among the different viral vectors, the use of wild-type and recombinant adeno-associated viral (AAV and rAAV, respectively) vectors has grown significantly. Production of AAV vectors at industrial scale requires the accurate measurement of the viral capsid concentration in both upstream and downstream AAV bioprocessing.
To meet increased throughput and safety demands, investigators are seeking more automated analytical solutions for assessment of viral vector identity, purity, and stability. While traditional approaches such as ELISA can be useful, they are often complex, time consuming, and nonstandardized. Octet® Bio-Layer Interferometry (BLI) systems offer a robust alternative to conventional ELISA by eliminating the hands-on steps that come with running a traditional immunoassay. Time is significantly decreased, and human error is reduced, too. Both of these factors adversely impact assay reproducibility and team productivity.
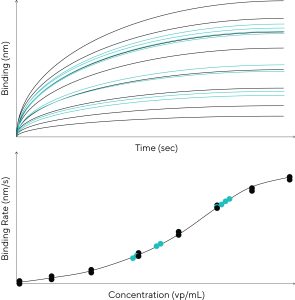
The utilization of Octet® AAVX Biosensors for the quantitation of multiple AAV serotypes using a simple, direct quantitation assay design on the Octet® Bio-Layer Interferometry (BLI) label-free detection platform is shown in Figure 1. The latest application note by Sartorius discusses key features and benefits of the Octet® AAVX Biosensor exemplified by its ability to bind to 10 different AAV serotypes (AAV 1–9 and AAVrh10), ability to quantitate both purified and crude viral samples, and good regenerability and re-use which helps reduce cost per assay.
Find out how colleagues at BridgeBio Pharma, and RegenXBio take this novel approach to AAV capsid titer quantification by using Octet® AAVX Biosensors to generate viral titer assay data that is highly correlated with corresponding AAV titration ELISA data. Binding a wide selection of AAV serotypes, the data proofs how the superior specificity ensures reliable AAV quantification. Excellent assay sensitivity and highly reproducible results were demonstrated, which help ensure the collection of the high-quality data required to meet regulatory standards for AAV titration throughout viral vector production workflows. The broad dynamic range of detection, between 8.5 x 108 to 1.0 x 1013 vp/mL, achievable with label-free viral titer assays is particularly useful for AAV quantification, as samples need fewer dilutions to fall within the quantitative range of the assay.
Octet® AAVX Biosensors can be regenerated and re-used up to 20 times, providing an efficient and cost-effective solution for high-throughput AAV quantitation applications. Furthermore, the Octet® BLI platform is available in a 21 CFR Part 11–compliant version, making it an ideal tool for industry environments where a good manufacturing practice (GMP) system is in place. All in all, new Octet® AAVX Biosensors fulfill the demands of timely data generation for maintaining efficient production and overcomes the lengthy investigations and sample retests.
Reference
learn more www.sartorius.com/octet-aavx-biosensor