Escherichia coli is widely considered the most cost-effective bacterial system for recombinant protein expression. Consequently, it is broadly used. Nonetheless, it has its limitations as an expression system. For example, it has difficulty secreting certain proteins, and it may necessitate a complicated series of procedures for reducing endotoxins. To overcome these and other limitations, GenScript has established a Bacillus subtilis expression platform to produce recombinant proteins with extremely low endotoxin levels, providing an alternative solution for secreted protein expression.
B. subtilis, a gram-positive soil bacterium, has been studied thoroughly, and its genome has been completely sequenced. Almost all its essential genes and metabolic pathways have been well investigated. B. subtilis is widely used in production of recombinant proteins in agricultural, medical, and food biotechnology, as well as in the production of prokaryotic industrial enzymes (such as laundry enzymes). In fact, approximately 60% of commercially available enzymes are produced from Bacillus species.
This host system for recombinant protein production has many attractive features:
- Lack of pathogenicity: B. subtilis is a nonpathogenic organism and is classified as biological hazard biosafety level 1.
- Insignificant bias in codon usage: There is no significant bias in codon usage for protein expression in B. subtilis, which allows researchers to test genes of interest in both E. coli and B. subtilis and compare the results in parallel.
- Superior secretory capacity: B. subtilis is capable of secreting functional extracellular proteins directly into the medium via the Sec and Tat pathways. Proteins so secreted can be directly harvested from the culture medium, which makes downstream purification relatively easy to handle.
- Short growing time: The growth time B. subtilis needs to reach the desired density for protein expression is just a few hours, which makes large-scale industrial processes feasible.
- Endotoxin-free expression: Lipopolysaccharides, commonly found in the outer membrane of gram-negative bacteria, contribute to endotoxin levels after cell lysis.
B. subtilis has only one cell membrane, which is lacking in lipopolysaccharides. Recombinant proteins expressed in B. subtilis are free of endotoxins, which helps advantage this organism over E. coli as an expression system. In addition,
B. subtilis is classified as a GRAS organism.Over the past several years, consistent efforts have been made to optimize and improve protein expression systems using
B. subtilis. In this tutorial, we will show how B. subtilis improves on the yield of proteins that are hard to express in E. coli, increases protein yield by taking advantage of the capacity to “secrete” into its medium, and produces low endotoxin levels without requiring additional endotoxin removal steps.
Materials and methods
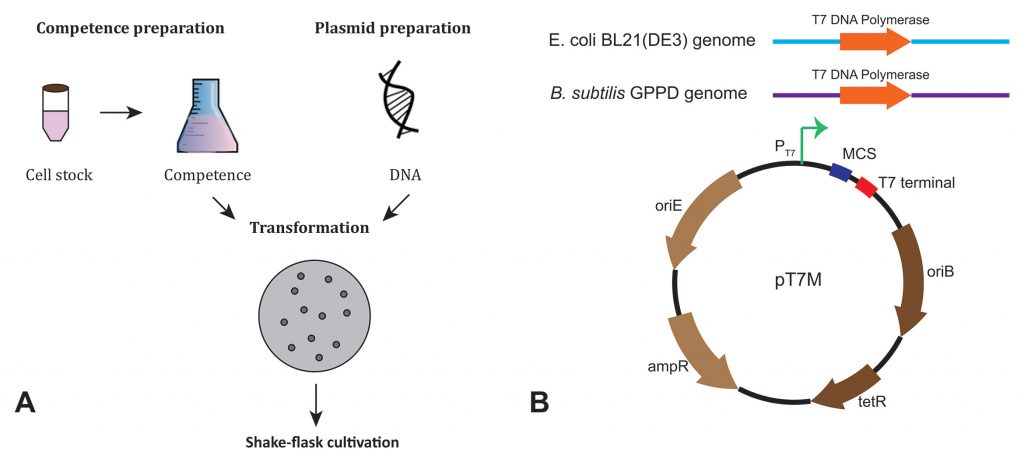
The steps for protein expression in B. subtilis are similar to those for E. coli. An expression plasmid is transformed into competent cells, which are then grown on antibiotic screening plates. After clones of interest are picked from the plate, the cells are cultivated in a shake flask.
The T7 RNA polymerase system for regulated and promoter-specific gene expression was adapted for and implemented into B subtilis. First, T7 RNA polymerase under the control of a xylose-inducible promoter was integrated into the B. subtilis genome, generating the B. subtilis GPPD vector. Then, the T7 promoter was inserted into a B. subtilis-E. coli shuttle vector, giving the resultant pT7M vector. pT7M can then be applied in both E. coli and B. subtilis for protein expression tests in dual expression systems, with isopropyl-β-D-thiogalactoside inducing E. coli BL21 (DE3) and xylose inducing B. subtilis GPPD (Figure 1).
Results
Several case studies are provided here to illustrate the performance of the B. subtilis expression system and the pT7M vector. For several proteins, quality and yield are described, and yield and endotoxin levels are compared.
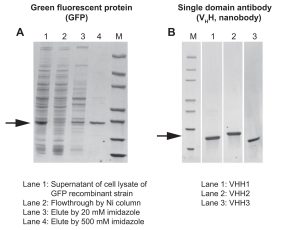
The first set of proteins tested in the B. subtilis expression system included green fluorescent protein (GFP) and three different single-domain antibodies (also known as sdAbs, VHHs, or nanobodies). In all cases, a protein purity level of >85% was obtained from one-step purification. Low endotoxin levels of 0.01 EU/µg were achieved without additional endotoxin removal steps (Figure 2).
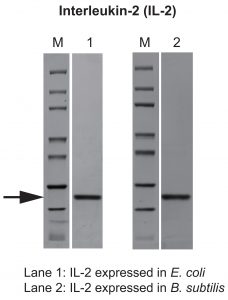
To compare the same protein produced across different bacterial expression systems, interleukin-2 (IL-2) was expressed in and purified from both E. coli and B. subtilis. Protein purity levels were the same (90%), and the yields were comparable for the two bacterial expression systems. The endotoxin levels of the IL-2, however, showed striking difference. The 0.008 EU/µg from B. subtilis was obtained without any additional endotoxin removal steps; however, even with two additional endotoxin removal purification steps, the endotoxin level from the E. coli system was more than three times higher. These data demonstrate the benefit of low-endotoxin protein production from a B. subtilis expression system (Figure 3).
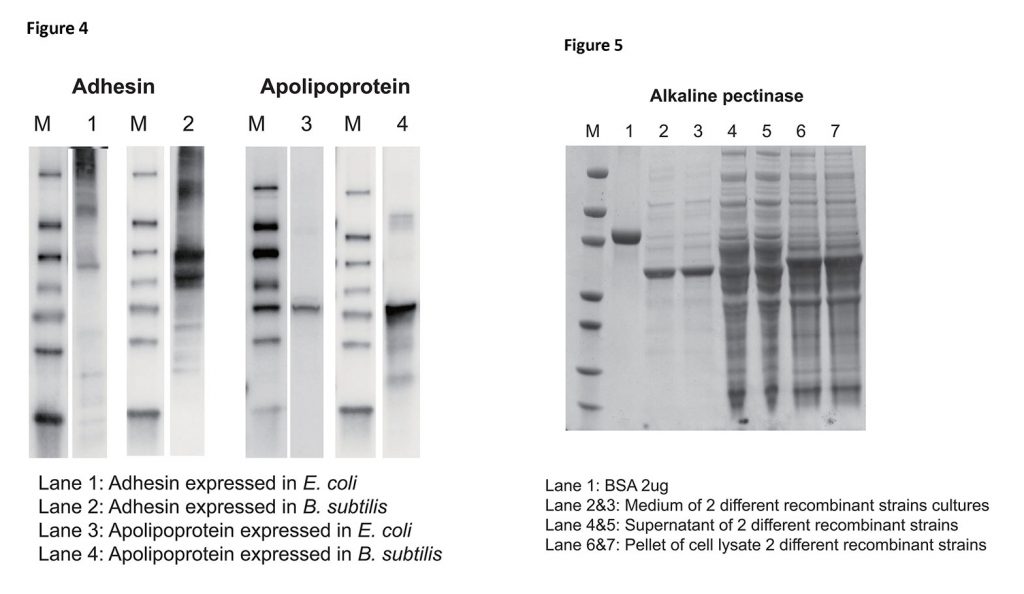
For proteins that are difficult to express in other bacterial expression systems, B. subtilis can be a better alternative for increasing expression levels. In the examples below, both adhesin and apolipoprotein exhibited a three- to fivefold higher expression level in B. subtilis than the expression level in E. coli for the same protein (Figure 4).
As for secreted proteins, high protein yield can be easily achieved in B. subtilis by harvesting secreted proteins directly from the culture medium. For example, alkaline pectinase forms exclusion bodies (aggregates) when expressed in E. coli, which makes it difficult to produce in large quantities. Neither the pellet nor the supernatant yields any promising protein at levels suitable for subsequent purification. In contrast, a high yield of 200 mg/L was obtained from expression in B. subtilis by direct purification of the culture media (Figure 5).
Conclusion
In this tutorial, we described how the B. subtilis expression system was established and provided a few case studies to demonstrate the applications and value of using B. subtilis as a host expression system. B. subtilis provides an alternative system for proteins that are difficult to express in E. coli. The advantage of B. subtilis is that it can “secrete proteins directly into the culturing medium” and achieve low endotoxin levels for purified proteins without necessitating any extra endotoxin removal steps.
Bowu Luan, PhD, is a product manager and Sen Yang, PhD, is a senior scientist at GenScript.