Rowan Moore Vertical Marketing Manager Thermo Fisher Scientific
Martin Samonig Applications Scientist Thermo Fisher Scientific
Ken Cook Ph.D. EU Bio-Separations Expert Thermo Fisher Scientific
Alexander Schwahn European Support Expert Thermo Fisher Scientific
Mike Oliver Product Manager Thermo Fisher Scientific
Remco Swart Ph.D. Director Product Manager Thermo Fisher Scientific
Kelly Broster Pharma & Biopharma Vertical Marketing Manager Thermo Fisher Scientific
This is the fourth and final article in the series, describing the characterization of protein-based bio-therapeutics by bottom-up analysis at the peptide level (peptide-mapping analysis). This article summarizes the requirements for mass spectrometry.
Advancements in simplicity, speed, throughput, and high levels of reproducibility are offered with new sample preparation approaches (e.g., Thermo ScientificTM SMART DigestTM Kits) and UHPLC systems (e.g., Thermo ScientificTM VanquishTM UHPLC system). These significantly improve the process and confidence in results for peptide mapping workflows. The final stages of the workflow involve detection, processing, and data interpretation, where high resolution accurate mass (HRAM) mass spectrometry provides a fundamental function in characterizing the protein of interest.
HRAM peptide mapping provided by the Thermo ScientificTM OrbitrapTM based MS platforms allows for fast, precise, and reproducible results which facilitates primary level bio-therapeutic characterization with confidence. HRAM Orbitrap LC-MS and LC-MS/MS systems provide precise mass measurements for each peptide, which is imperative in elucidating the correct peptide map. Lower resolution mass spectrometry systems have the potential for incorrect identification, which could have a significant impact on the results obtained. The example in Figure 1 highlights the advantages of using higher resolution MS systems, such as the Orbitrap system, to provide greater confidence in results. As can be seen, with a lower resolution incorrect identifications can be obtained.

Figure 1. Comparison of different high resolution systems for correct identification of peptides. RP = resolving power. Ref: Joshua J. Coon, et al. ASMS 2012 oral
Further certainty in results can be obtained by fragmentation of the individual peptides. Hybrid quadrupole-Orbitrap based LC-MS/MS instruments allow each peptide to be isolated and further fragmented, using higher-energy collisional dissociation (HCD) in the gas phase, which induces fragmentation of peptides along their backbone producing signature fragment ions. The MS precursor ion spectra reveal the accurate ion masses of the peptides and MS/MS fragment ion spectra reveal the b- and y-ion amino acid fragments of each peptide, and thus information on the modifications that are present (Figure 2). Both MS and MS/MS data can then be used for identification of peptides, facilitating identification of very small peptides and disulfide-linked peptides, which would be missed by MS/MS only.
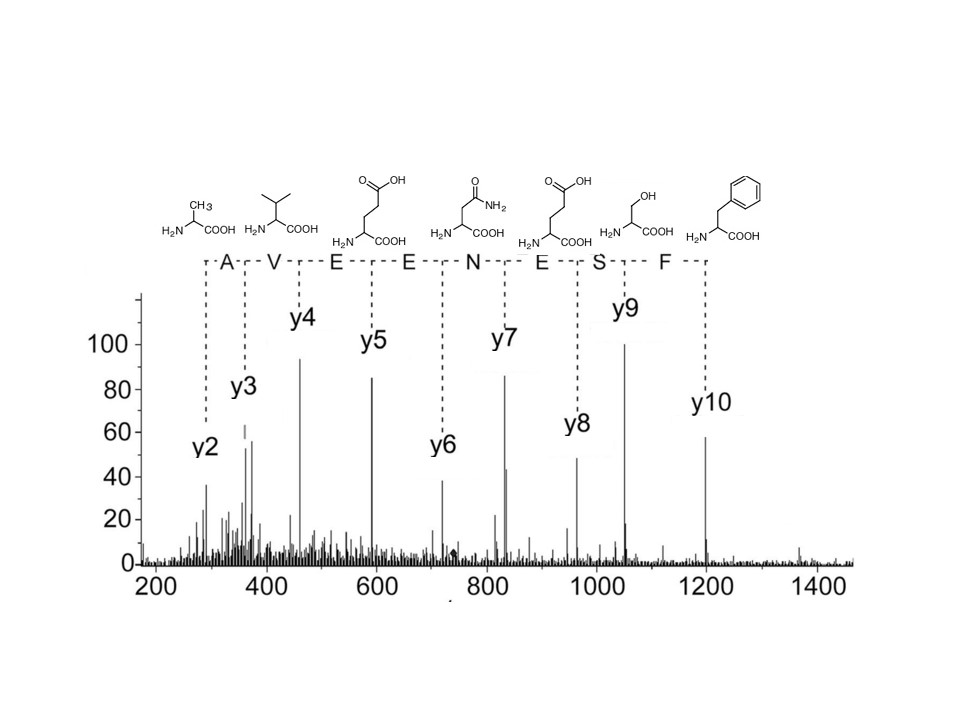
Figure 2. Example of peptide MS/MS mass spectrum showing amino acid fragment ions.
However, mass accuracy and the ability to fragment the ion of interest is only one requirement for mass spectrometers used in peptide mapping workflows. The use of UHPLC systems, such as the Vanquish Horizon and Flex UHPLC systems, help provide improved separations for these complex peptide sample sets combined with fast run times to aid throughput. As a consequence peak widths are often <5 seconds, which then requires MS systems which can scan fast enough to ensure collection of high quality MS and MS/MS data. The scan speeds achievable using Orbitrap-based instruments have been demonstrated to exceed the demand for even the most rapid UHPLC separation protocols. A data dependent acquisition analysis incorporating MS/MS analysis of the top 5 most intense MS peaks during a rapid 5 minute UHPLC gradient with typical chromatographic peak width of 2.4s resulted in a sum of 31 scans per peak (Figure 3).

Figure 3. Rapid instrument scan speed for fast peptide mapping.
Once data has been collected, it needs to be automatically processed and the output needs to allow in-depth data interpretation software solutions, such as Thermo Scientific™ BioPharma Finder™ software, seamlessly provide comprehensive results, including amino acid sequence confirmation with mass accuracy, modification, identification, retention time, and confidence information. The data is shown in multiple displays to enable effective data mining for features such as sequence coverage maps (Figure 4) and chromatographic peak shading, showing relative quantitation of co-eluting peptide peak contributions (Figure 5).

Figure 4. Sequence coverage map of the heavy (right) and light chain (left) of Rituximab, with 100% sequence coverage. Processed using the BioPharma Finder software.
Peptide mapping is essential for bio-therapeutic characterization. Fundamental to the success of these studies is the reproducibility of the workflow, which enables users to confidently assign data differences to the sample and not the methodological conditions employed. Reproducibility is influenced by protein digestion, chromatographic separation, detector performance, and linearity and consistency in data handling. A comprehensive peptide mapping workflow with standardization of the various steps of the process improves peptide mapping reproducibility and increases analytical confidence.

Figure 5. Chromatographic peak shading showing relative quantitation of co-eluting peptide peak contributions. Processed using the BioPharma Finder software.
Resources:
1. SMART Digest and SMART Digest ImmunoAffinity (IA) Technical Guide
2. Vanquish UHPLC System Built for Biopharma Brochure
3. Application Note: LC-UV-MS Peptide Mapping Development for Easy Transfer to LC-UV QA/QC.
4. Q Exactive BioPharma Mass Spectrometer Brochure
The Thermo Fisher Scientific contributing authors include Martin Samonig, application scientist, LC-MS; Alexander Schwahn, European support expert for biopharma industry; Ken Cook, EU bio-separations expert; Mike Oliver, product manager, sample preparation and Accucore LC products; Remco Swart, director and product manager, HPLC & LC-MS solutions; Rowan Moore and Kelly Broster, marketing managers for the pharma and biopharma vertical.



