February 1, 2015 (Vol. 35, No. 3)
Focus on Preparing Buffers and Media for Preclinical Batches through to 5,000 L Scale
Mixing of buffers and media in a GMP biologics manufacturing setting is critical to ensuring that cells have the correct bioprocess conditions for growth and production of biologics, vaccines, and cell therapies, as well as the correct media and downstream purification buffers.
If buffers and media are not homogeneously mixed then pH, nutrient, and gas supply may be suboptimal for cell growth, cell viability, and protein expression, and downstream purification steps may function sub-optimally. This can lead to problems with reduced yields of the biological or cell therapy product, which can cost a significant amount in lost production.
Traditionally, because of the scale of buffer and media preparation required in biologics manufacturing, these liquids-liquids or liquids-powders are mixed in fixed stainless-steel tanks using an impeller to produce a uniform solution. With stainless-steel mixing tanks, there is a risk of cross-contamination of buffers and media, so the tanks require cleaning and sterilizing between runs, as well as validation of the cleaning and sterilizing process. These tasks take additional time and increase the costs of each process run.
These cleaning and validation issues, coupled with the time-saving efficiencies single-use chambers provide, have led to the commercial development and increasing adoption of single-use buffer and media preparation chambers. The mixing systems used in these single-use chambers vary from stirrer rods or wands at the 20–200 L scale through to more efficient magnetic impellers at larger scale (20–2,000 L).
At manufacturing scale, however, the most commonly used magnetic impeller systems in single-use chambers utilize impellers in which cylindrical jets spread out from the impeller to create radial flow patterns. Some of the liquids and solids being mixed travel up the bag wall, and some of them travel down the walls, making this method limited in its mixing efficiency. Therefore, many single-use mixing chambers scale to only 2,000 L, as the impeller types used are not capable of sufficiently mixing larger buffer and cell media volumes.
An additional disadvantage with using impeller mixing is that it generates particulates and high sheer forces, which may damage more delicate media components, and this can cause problems with cell therapy media, for example.
As a result, there remains a need for single-use mixing technology that is robust enough to homogeneously mix even viscous liquids. This platform should scale from 30 L to beyond 2,000 L and must also be capable of mixing without generating damaging high sheer forces.
imPULSE Mixing Platform
ASI-Life Sciences recently introduced the imPULSE single-use mixing system (Figure 1), which exclusively utilizes single-use flexible chambers in sizes from 30 L to 5,000 L. The system was developed with the following objectives in mind: robust mixing for a variety of different media viscosities, full scalability between the entire size range, and low sheer forces.
The single-use chamber easily fits the imPULSE mixing vessel. Associated tube sets are available in a standard configuation, or they can be customized to meet the pharma, biotech, or contract research organization’s specific needs. Larger chamber sizes can be installed with the assistance of a bag loading cart, which is offered as an optional accessory.
The most critical part of the imPULSE mixing technology is the mixing action generated by the vertical movement of the mixing disc, which has slotted openings and hinged flaps that open on the upstroke and close on the downstroke of the mixing disc assembly. On the downstroke, with the flaps closed, a column of liquid is displaced under the disc, and it moves between the outside profile of the disc and the walls of the mixing vessel.
The ratios between the size of the mixing vessel, header size, height of the vessel, and the size of the mixing disc are maintained throughout the entire imPULSE portfolio to insure scalability. In addition, the mixing stroke height and speed can be adjusted to provide the best mixing profile for the application.

Figure 1. The imPULSE Mixing System
Mixing Application
The efficacy of liquid movement throughout the chamber in the imPULSE platform has been demonstrated in a heat-mapping study in which four thermocouples, spaced vertically over a 250 L mixing chamber, measured less than 1°C heat change when heating and mixing water from 19 to 49°C over 170 minutes.
In a second proof-of-concept study, homogenous solid-liquid (buffer production) and liquid-powder (cell culture media production) mixing was shown. For buffer production, phosphate-buffered saline (PBS) (approximately 2,500 g) added to water in a 250 L mixing chamber started to mix in seconds and was uniformly dissolved (measured by consistent conductivity, µS/cm) in 2 minutes, and sodium chloride (NaCl) (2,000 g) added to corn syrup in a 250 L single-use chamber began to mix in 30 seconds and was uniformly dissolved in under 5 minutes (measured by µS/cm).
To mimic cell media production, bovine serum albumin (BSA) (250 g) added to water in a 250 L mixing bag started to mix in water at 30 seconds and was also fully mixed (measured by optical density at 280 nm) in 4 minutes.
These results show the uniform nature of water flow that the mixing disc creates and also indicates that the action of the disc can efficiently and rapidly aid in dissolving salts and dispersing powders (which tend to float and resist hydration) in low and medium viscosity solutions.
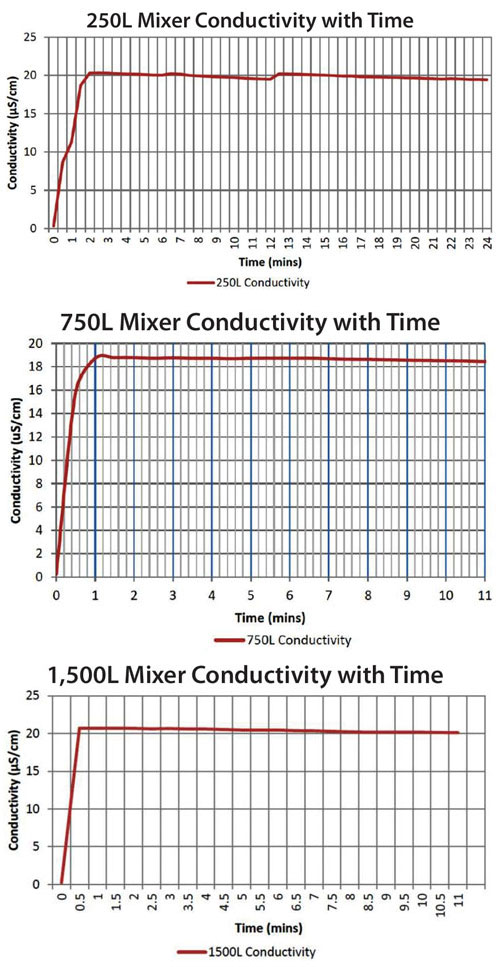
Figure 2. Conductivity of PBS in imPULSE single-use mixing chambers of 250 L, 750 L, and 1,500 L
Scalability
Scalability of the mixing technology was demonstrated in a study where PBS was added to water in mixing chambers of increasing sizes. PBS (approximately 2,500–15,400 g) started to dissolve in water in seconds and was uniformly dissolved (measured by µS/cm) in under 2 minutes in mixing bags of 250 L, 750 L, and 1,500 L (Figure 2).
Additionally, to show scalability of the technology up to 5,000 L, computational fluid dynamics (CFD) models for 250 L, 1,500 L, and 5,000 L were generated applying a mixing disc velocity of 0.1 m/sec. The model predicted that at 6 seconds, in any of the three vessels, salt would have moved to the top (or near the top) of the chamber. Also, a large amount of the salt would have started to dissolve into the liquid (Figure 3).
The speed of mixing in the 250 L and 1,500 L model is supported by the experimental study evidence previously discussed, and since the 5,000 L model shows a similar pattern of mixing to the 250 L and 1,500 L models, this indicates that mixing of buffer salts in a real 5,000 L single-use bag using the imPULSE mixing disc should also be uniform within minutes.
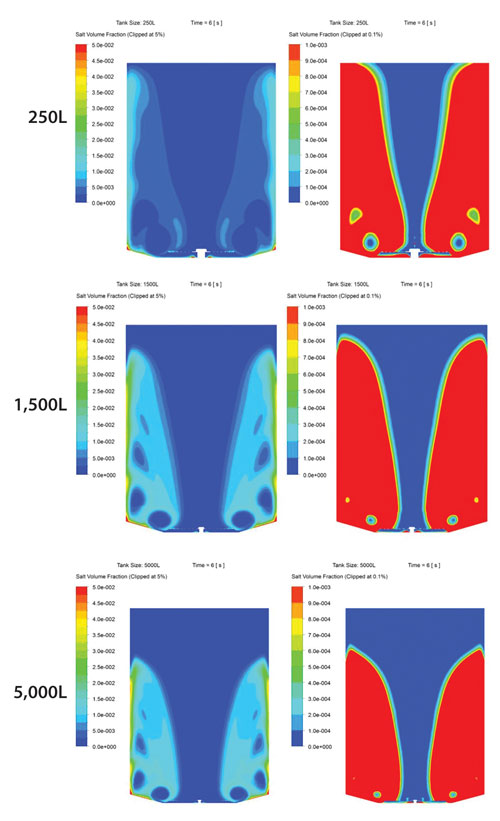
Figure 3. At right, CFD-generated models of salt volume fraction contours at the 6 second time point in imPULSE platforms of 250 L, 1,500 L, and 5,000
Conclusion
The application of a single-use mixing system eliminates a number of sterilization steps that are required when using conventional stainless-steel infrastructures. Closed single-use systems offer many advantages including: speeding up the process application change, reducing the risk of contamination in a mixed media, and providing a cleaner environment.
A single-use system supplier needs to be selected carefully since the FDA’s strict validation requirements demand significant resources in direct cost and time. Any changes in single-use system design or use of construction materials can require a costly and time-consuming revalidation.
The implementation of the imPULSE mixing platform improves workflow and contributes to faster bioprocess development of biologics, vaccines, and cell therapies.
Rudolf Pavlik ([email protected]) is director of product development-process equipment at ASI-Life Sciences. For additional information, contact Amber Sherrick ([email protected]), marketing manager, ASI-Life Sciences.


