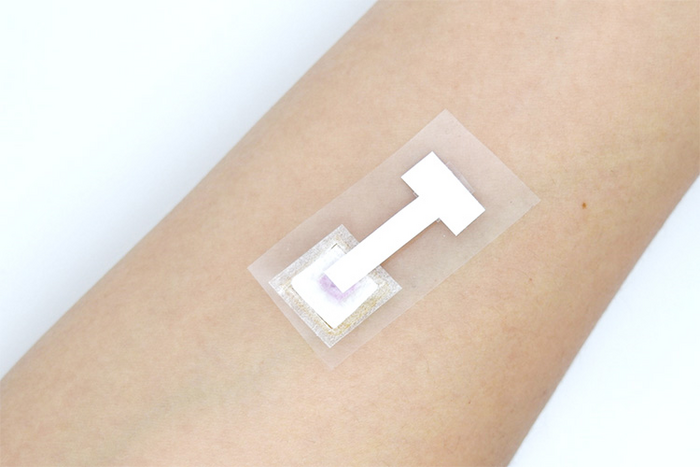
A rapid and reliable skin-patch test1 can now detect the COVID-19 virus, and potentially other infectious agents in under three minutes, without the need to draw blood. This convenience overcomes a current challenge in identifying infected individuals who are averse to blood tests and could help restrict the spread of the pandemic.
Asymptomatic individuals constitute 16–38% of the SARS-CoV-2 infected population which increases the difficulty of identifying infected individuals. The lack of convenient and sensitive tests to detect the virus in all individuals is continuing to limit global response to the pandemic.
Predominantly, SARS-CoV-2 is detected through RT-PCR (real-time reverse transcription polymerase chain reaction) on swab samples collected from the nose and throat. However, these tests require long detection times, high costs, specialized equipment and medical personnel, and are not feasible in areas where resources are limited.
Alternatively, COVID-19 infection is detected through antibody tests (immunoassays) using blood samples collected from finger pricks by a lancing device. These popular point-of-care options require gold nanoparticle-based testing strips and involve risks of cross contamination and biohazard.
Immunochromatographic tests that detect anti-SARS-CoV-2 immunoglobulin M (IgM) and immunoglobulin G (IgG) can provide clinically relevant information regarding the course of COVID-19 infection, but invasive blood sampling poses a major obstacle.
“To develop a minimally invasive detection assay that would avoid these drawbacks, we explored the idea of sampling and testing interstitial fluid, which is located in the epidermis and dermis layers of human skin,” said first author Leilei Bao, PhD, researcher at the Institute of Industrial Science, at The University of Tokyo. “Although the antibody levels in the interstitial fluid are approximately15–25% of those in blood, it was still feasible that anti-SARS-CoV-2 IgM/IgG antibodies could be detected and that interstitial fluid could act as a direct substitute for blood sampling.”

“We developed biodegradable porous microneedles made of polylactic acid that draws up the interstitial fluid from human skin,” said Beomjoon Kim, PhD, professor at the department of mechanical and biofunctional systems at The University of Tokyo, and the senior author of the paper. “Then, we constructed a paper-based immunoassay biosensor for the detection of SARS-CoV-2-specific antibodies.”
Dermal interstitial fluid is a rich and accessible source of protein biomarkers, including antibodies. The authors prepared polylactic acid microspheres from a single emulsion to form continuous micropores, which they then heated for half an hour at 180°C to bind them together. They demonstrated these microneedles fabricated with emulsion droplets could effectively penetrate and extract interstitial fluid by capillary effect from rat and pig skin, used to model human skin.
The extracted interstitial fluid flows vertically into the attached nitrocellulose paper biosensor, where virus-specific antibodies are detected visually through a colored-based reaction (colorimetry).
The researchers show that anti-SARS-CoV-2 IgM and IgG levels as low as 3 and 7 ng/mL, respectively, can be detected using the skin patch test which shows the advantage of this new method over current commercially available lateral-flow immunochromatographic assays (LFIA). The patch, 1.5 cm by 3.5 cm in size, detects anti-SARS-CoV-2 antibodies in the interstitial fluid in a painless and convenient test, in under three minutes.
The authors believe that the speed, safety, simplicity, convenience, and minimally invasive nature of the compact anti-SARS-CoV-2 IgM/IgG biosensor device will lead to its widespread use. In addition to COVID-19, the authors claim that the device can be customized to rapidly screen various infectious agents and provide a complementary diagnostic test.
References
Bao, L., Park, J., Qin, B. et al. Anti-SARS-CoV-2 IgM/IgG antibodies detection using a patch sensor containing porous microneedles and a paper-based immunoassay. Sci Rep 12, 10693 (2022).