Circular dichroism (CD) spectroscopy has long been regarded as a low-resolution technique used to obtain an estimate of the a-helix and b-sheet content of proteins. This view of CD, though widely held, is no longer accurate and is being replaced by the realization that modern CD spectroscopy systems offer enhanced resolution and thereby provide critical information regarding changes in both secondary and tertiary structural elements.
The potential for relatively minor changes in these higher order structure (HOS) elements to have a significant effect on biological activity, interactions, and stability, or on the efficacy and safety of a biotherapeutic, is well recognized. A reliable method for HOS comparison is an essential tool to ensure comprehensive biophysical characterization.
Setting Standards for HOS Comparisons
To provide scientists with a standard suitable for use with commonly used biophysical characterization techniques, the National Institute of Standards and Technology (NIST) at the U.S. Department of Commerce offers a specifically selected monoclonal antibody, NISTmAb.
Selection of a mAb as a biological standard reflects the importance of these proteins in both research and biotherapeutic development.
Figure 1 shows the secondary structure of the NISTmAb, as revealed by analysis in the far-UV. This data contributed to the initial characterization of the NIST antibody standard.
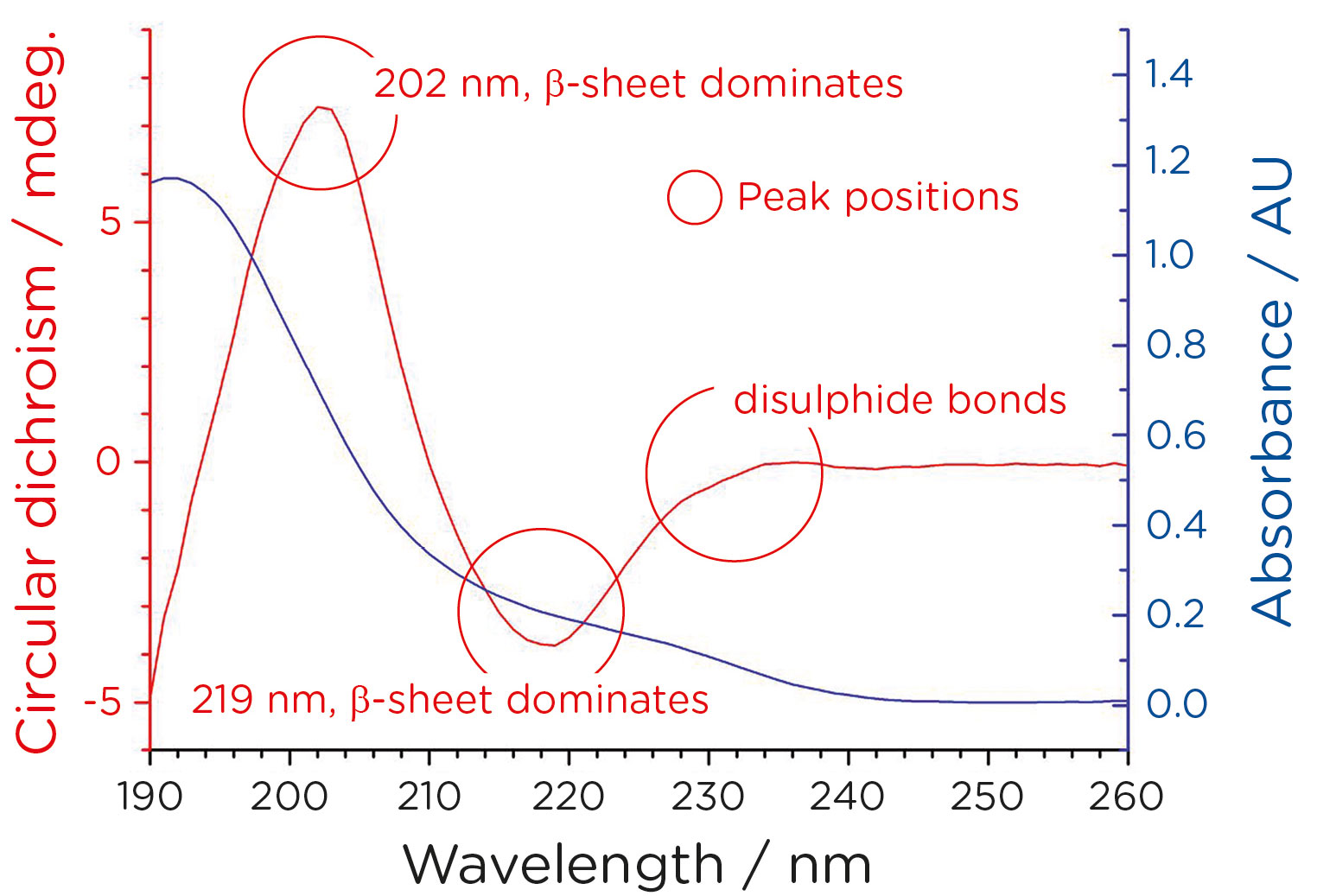
Meeting Changes to Regulatory Demands
During biotherapeutic development, many biophysical characterization techniques are required to support informed decision making and contribute to the totality of evidence in regulatory submissions. As an essential part of this toolset, CD has been referred to in more than 95% of biosimilar applications involving mAbs and other biotherapeutics. This figure was cited by Maria Teresa Gutierrez Lugo, Ph.D., Office of Biotechnology Products, Center for Drug Evaluation and Research (CDER), Food and Drug Administration (FDA), at the Fifth International Symposium on Higher Order Structure of Protein Therapeutics, in a talk entitled “Regulatory Consideration for the Characterization of HOS in Biotechnology Products.”
However, regulatory authorities are increasing their demand for state-of-the-art techniques that provide “statistically validatable data.” Until now, obtaining such results for HOS comparisons has presented challenges in terms of data acquisition and suitability of statistical methods.
With the introduction of an automated and integrated solution for CD analysis that eliminates the errors associated with manual sample handling, and thereby ensures the reproducibility essential for multiple repeat analyses, high-quality CD data has become amenable to statistical interrogation. This allows for a more rigorous, objective assessment when looking for even minor changes in the HOS of complex biomolecules.
HOS Comparisons During Biotherapeutic Development
To ensure an effective, efficient workflow, detecting and confirming minor differences upstream is as important as proving similarity downstream. Consequently, an ability to detect minor changes in HOS is crucial throughout development and scale-up of innovator drugs or potential biosimilars.
The secondary and tertiary structures of commercially available innovator lots were compared with test batches of a potential biosimilar.
Analysis in the near-UV (Figure 2) revealed the possible presence of minor differences in tertiary structure. However, any conclusions drawn by visual comparison of the CD spectra are likely to be subjective.
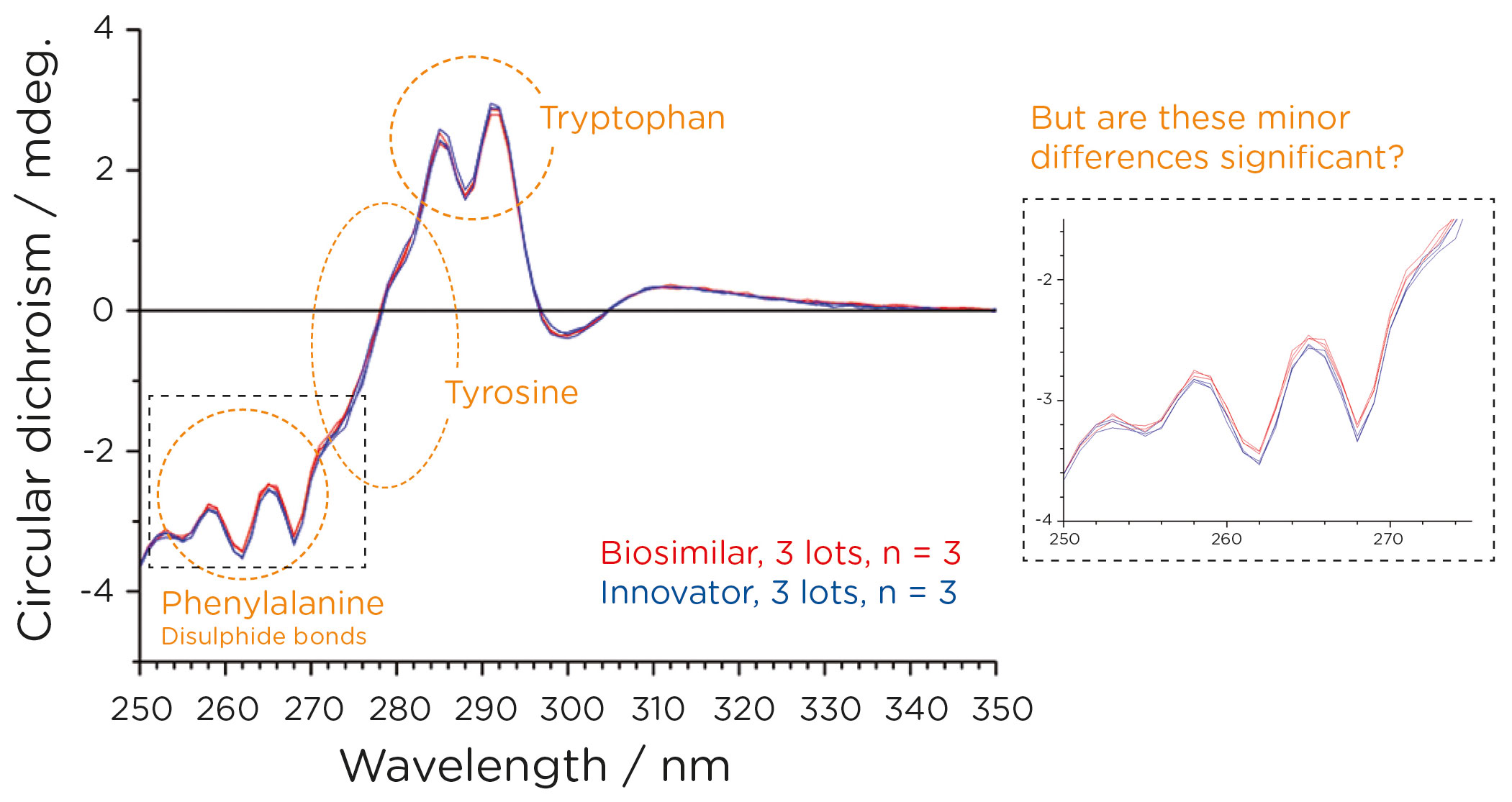
To facilitate an objective conclusion, CD spectral data were converted into a numerical format using the weighted spectral difference method (Dinh, N.N., et al. Anal. Biochem. 2014; 464: 60–62) incorporated into the Chirascan HOS Comparison software. This method for “spectral conversion” was recommended in a recent FDA publication, “Statistical approaches to evaluate analytical similarity—guidance for industry”. The numerical data generated was used to apply the Tier 2 quality range test as recommended by the Office of Biostatistics and the Office of Biotechnology Products, CDER/FDA (Figure 3).
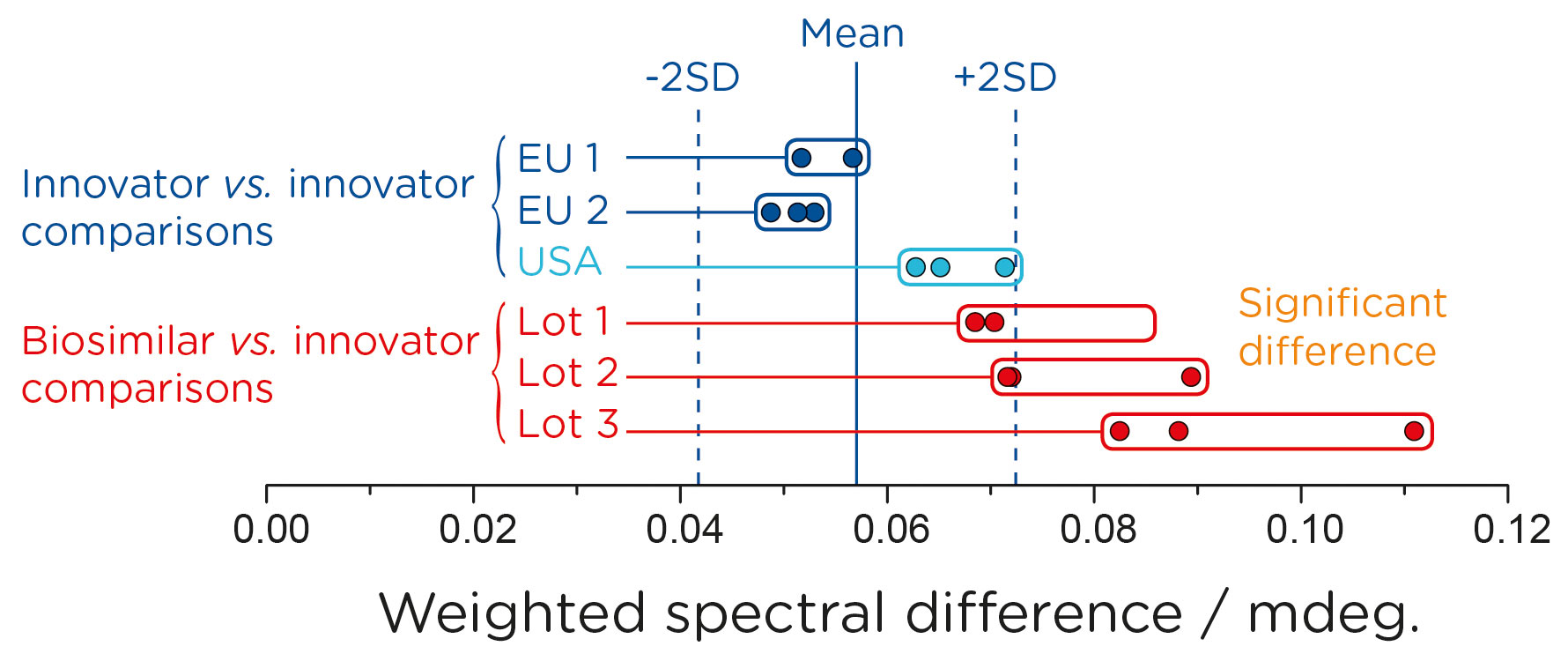
The CD analysis was sufficiently sensitive that statistical analysis of the raw data, using ±2 SD acceptance criteria, revealed not only statistically significant differences between the biosimilar batches and the innovator, but also differences between innovator lots manufactured at different geographical locations.
Applying the same analytical approach to comparisons of secondary structure showed no statistically significant changes between innovator and biosimilar lots (data not shown).
Conclusion
CD analysis has now become a technique that enables objective, statistical quantification of differences and similarities in HOS. The results generated will contribute significantly to the effectiveness of comparability programs and support informed decision-making throughout biotherapeutic development and scale-up.
In addition, establishing the statistical significance of minor changes under native and stressed conditions will facilitate the definition of an acceptable range for HOS variability within any control strategy for safety, quality, efficacy, and manufacture.
Ultimately, the ability of a new generation of CD systems to generate high-quality, statistically validatable HOS comparisons will strengthen the totality of evidence required for successful regulatory submissions.
Circular Dichroism— A Brief Reminder
