August 1, 2011 (Vol. 31, No. 14)
Integral Molecular Uses Shotgun Mutagenesis to Germline-Humanize Monoclonal Antibodies
Seventy percent of FDA-approved monoclonal antibodies (mAbs) and approximately 66% of mAbs currently in clinical trials were originally derived from nonhuman species. Typically, such mAbs are humanized during clinical development to minimize the potential of nonhuman sequences to elicit unwanted and potentially dangerous immune responses in patients.
Humanization, however, represents a challenge for antibody engineers, since immunogenicity stems not only from foreign sequences, but also from unique sequences derived by the natural process of VDJ recombination and affinity maturation, or inadvertently by antibody engineering (e.g., to improve stability or manufacturing).
Even fully human mAbs (i.e., derived directly from a person) can elicit unwanted human antihuman antibody (HAHA) responses. For example, adalimumab (Humira), a fully human antibody against TNF, shows marked HAHA responses in up to 89% of patients. Understanding, controlling, and engineering antibodies to bypass potential immunogenicity is therefore of significant interest for the development of safer antibody therapeutics.
This tutorial reviews several humanization strategies and presents a new approach for creating maximally humanized mAbs.
Reducing mAb Immunogenicity
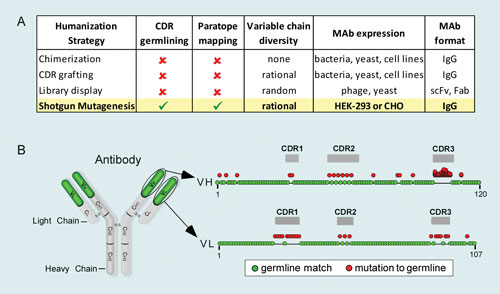
Several different experimental approaches are being used for humanizing mAbs. Chimerization, introduced over 25 years ago, recombines mouse variable chains onto human Fc scaffolds. Chimerization has been used to generate five FDA-approved mAbs to date, including abciximab (ReoPro) and rituximab (Rituxan).
Chimeric mAbs have proper Fc effector function but can exhibit significant immunogenicity due to nonhuman sequences in the variable domains. To circumvent this, many labs have turned instead to CDR grafting, where the six complementarity determining regions (CDRs), rather than the full mouse variable chains, are transplanted onto a human IgG scaffold that includes human variable chain frameworks and constant chains. CDR grafting has been used to generate nearly half of all FDA-approved therapeutic antibodies, including trastuzumab (Herceptin), which elicits HAHA responses in
Despite looking more like human antibodies, some CDR-grafted mAbs (e.g., alemtuzumab (Campath)) elicit more significant immune responses due in part to murine CDR residues, not all of which contribute to antibody specificity. Moreover, CDR grafting can result in epitope drift, a change in antibody specificity due to subtle structural changes in the altered variable chains. Thus, even the best humanization approaches usually require additional engineering to introduce back mutations (reversions to murine origin) into antibody frameworks.
Fully human antibodies derived entirely of human sequences are typically less immunogenic than humanized counterparts and are an increasingly popular starting point using display technologies (phage, yeast, and ribosome), humanized mice, or human B cells. These approaches have been used to generate nine FDA-approved mAbs to date, including belimumab (Benlysta), the first new treatment for lupus in 50 years.
Despite being of human origin, more than half of these human mAbs elicit detectable immune responses in humans, while responses to many other fully human mAbs in clinical trials are unknown. One of the reasons for the immunogenicity of human mAbs is that large randomized libraries can result in the selection of hypermutated binders that may contain nonessential changes or foreign looking residues. Thus, even fully human mAbs often require further humanization to reduce potential immunogenicity.
Shotgun Mutagenesis
Addressing the need for antibodies that are maximally humanized, Integral Molecular is using its Shotgun Mutagenesis technology to comprehensively germline-humanize mAbs. This high-throughput protein-optimization technology substitutes every amino acid residue within the variable chains that diverges from consensus human germline sequences.
Antibody variants are engineered, expressed in human cells, and screened for antigen binding to comprehensively define all tolerated positions and determine the contributions of individual residues to antibody binding. This approach differs from conventional approaches in that it includes substitutions within the CDRs, which are usually not mutated using other approaches due to the risk of disrupting antigen interactions (Figure 1A).
Shotgun Mutagenesis uses human cellular expression to ensure proper folding and post-translational modifications and production of whole IgG molecules to assay antibodies directly in their final format.
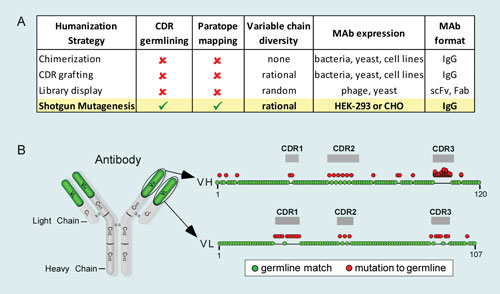
Figure 1. Shotgun Mutagenesis maximal antibody humanization: (A) Comparison of Shotgun Mutagenesis to conventional humanization strategies. (B) All palivizumab residues in the variable chains (frameworks and CDRs) that deviate from human germline were individually mutated (red circles) to corresponding residues from human germline sequences. Residues in CDR3 of VH were comprehensively mutated to account for several possible JH and D sequences. VH=variable heavy chain, VL=variable light chain.
Humanization of Palivizumab
Integral Molecular has applied its Shotgun Mutagenesis technology to maximally humanize the FDA-approved antibody palivizumab (Synagis). Palivizumab is a wellstudied mAb that binds the F protein of respiratory syncytial virus (RSV) and is used for the prevention of respiratory tract disease in children. Palivizumab was originally derived from a mouse antibody and was subsequently humanized by CDR grafting onto a human framework. Although palivizumab contains 95% human sequences overall, 20% of the residues within its variable chains and 58% of the residues within its CDRs differ from human germline sequence.
To maximally humanize palivizumab, sitedirected mutations were individually introduced into each position of the variable chains, including all six CDRs, which differed from consensus germline sequences (Figure 1B). A total of 93 palivizumab variants were engineered, expressed in human cells, and assayed for binding to full-length RSV F protein. Many of the mutants showed near wild-type binding, demonstrating that these positions could readily tolerate a germline substitution (Figure 2A).
A second class of mutants showed positive but reduced binding, suggesting that germlining at these positions comes at the expense of antigen binding (Figure 2B). A third class of mutations completely abrogated binding, indicating the requirement of these residues for interacting with RSV F (Figure 2C). A small number of palivizumab mutants, including several reported in the literature, showed enhanced binding, which could be used to further optimize palivizumab for higher affinity.
Mutations representing each binding class were mapped and visualized onto the crystal structure of a palivizumab derivative (Figure 2D-F). As expected, palivizumab mutants that failed to bind RSV F contained substitutions in residues that are in close contact with the antigen. These mutations are of particular interest because they provide insight into palivizumab’s paratope, the portion of the antibody that is required for binding its target.
The ability to identify mutations that abrogate antigen binding enables the determination of all residue positions amenable to humanization (i.e., nonessential residues). Remarkably, 25 out of the 45 positions tested in the variable chains could tolerate the introduction of a human germline residue. Thus, even previously humanized mAbs can still be further maximally humanized in order to improve their safety profile.
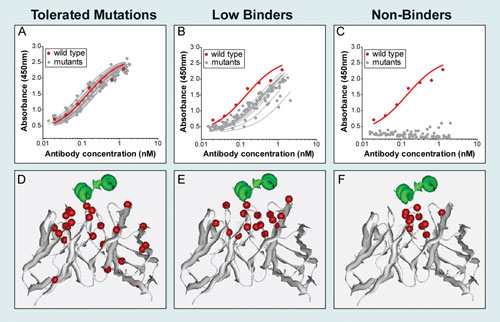
Figure 2. The effect of germline substitutions on palivizumab binding: Individual germline palivizumab mutants were evaluated by ELISA for binding to full-length RSV F protein incorporated into Lipoparticles (virus-like particles) and compared to wild-type palivizumab (red trace). Antibody variants were classified as (A, D) tolerated, (B, E) low binders, and (C, F) nonbinders, and positions of mutations (shown in red) were subsequently mapped onto the structure of a palivizumab derivative co-crystallized with a peptide from RSV F (shown in green).
Eli Berdougo, Ph.D ([email protected]), is a communications scientist, Joseph R. Couto, Ph.D., is the director of antibody development, and Benjamin J. Doranz, Ph.D., is president and CSO at Integral Molecular.