Polarized epithelial cells form barriers that separate the inner and outer layers of various organs such as kidney, colon, lung, and mammary glands. Epithelial barriers are essential for controlling passage of nutrients, hormones, gases, and cells between different parts of the body; thus, the ability of epithelial cells to polarize and form a barrier plays an important role in organ function. In the body, the basement membrane is responsible for helping epithelial cells to establish the correct polarity.
This process can be modeled and assayed in vitro by culture of epithelial cells in an extracellular matrix. Corning® Matrigel® matrix has been demonstrated to enable polarized epithelial structures to form in vitro and can be used to create a wide variety of 3D models such as kidney cysts for studying polycystic kidney disease. To increase the throughput of generating 3D models, Corning has developed 96- and 384-well microplates that are precoated with Corning Matrigel matrix. The current study highlights the use of Corning Matrigel matrix 3D plates to generate Madin-Darby canine kidney (MDCK) cysts for screening modulators of forskolin-induced cyst swelling, which can be used to screen potential therapies for diseases such as polycystic kidney disease.
MDCK cyst culture
MDCK cells (ATCC CCL-34) were cultured in Dulbecco’s Modified Eagle Medium (Corning Cat. No. 10-013-CM) containing 10% fetal bovine serum (Corning Cat. No. 35-010-CV). Twenty-four hours prior to use, Corning Matrigel matrix 3D plates (Corning Cat. No. 356256) were thawed while they were kept at 2–8 °C. On the day of seeding, plates were transferred to 37 °C to polymerize for at least 1 hour. While plates were polymerizing, cells were harvested with Accutase (Corning Cat. No. 25-058-CI) and resuspended at a density of 12,500 cell/mL supplemented with Corning Matrigel matrix (Corning Cat. No. 356231) to a concentration of 0.2 mg/mL. Once plates were polymerized, 40 µL of cell suspension was added to each well, and plates were incubated for 96 hours.
MDCK cyst staining
Cysts were collected from Matrigel matrix by pipetting up and down with wide-bore tips (Corning Cat. No. TF-205-WB-R-S) and incubating with Corning cell recovery solution (Corning Cat. No. 354253) at 4 °C for 20 minutes. Cysts were washed several times with cold phosphate-buffered saline before fixing with cold 4% paraformalde for 15 minutes at 2–8 °C. After fixation, cysts were permeabilized with 0.5% Triton™ X for 20 minutes prior to
washing with phosphate-buffered saline and staining.
Cysts were stained overnight at 2–8 °C in 200 µL of flow cytometry staining buffer (R&D Systems Cat. No. FC001) containing one drop of NucBlue Fixed Cell ReadyProbes Reagent (Thermo Fisher Cat. No. R37606), 2 µL of ZO1 (Thermo Fisher Cat. No. 339188), and 5 µL of phalloidin 647 (Thermo Fisher Cat. No. A22287) (Figure 1).
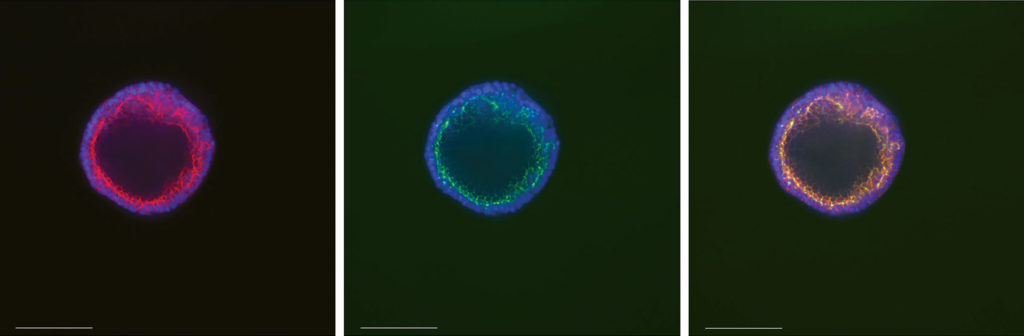
Swelling Assays
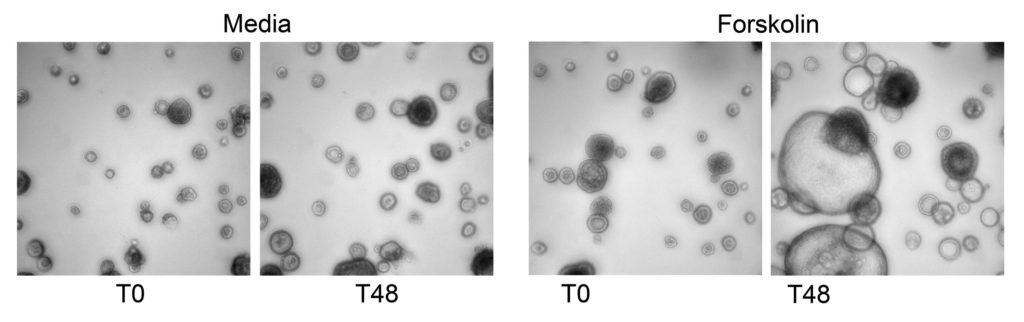
Z′ factor: After 96 hours, 40 µL of media containing 20 µM forskolin (MilliporeSigma Cat. No. F6886) or DMSO (Corning Cat. No. 25-950-CQC) control was added to each well. A time-zero scan with the Thermo Fisher CellInsight CX7 confocal imager was taken, and then plates were incubated for an additional 48 hours prior to scanning again (Figure 2).
Screen: Compounds from the Tocris kinase library (Tocris Cat. No. 3514) were added to MDCK plates to achieve a final concentration in each well of 10 µM forskolin and/or 10 µM compound or media alone. DMSO concentration was matched in all wells to 0.3%. Plates were imaged prior to compound addition and again after 48 hours of incubation (Figures 3 & 4).


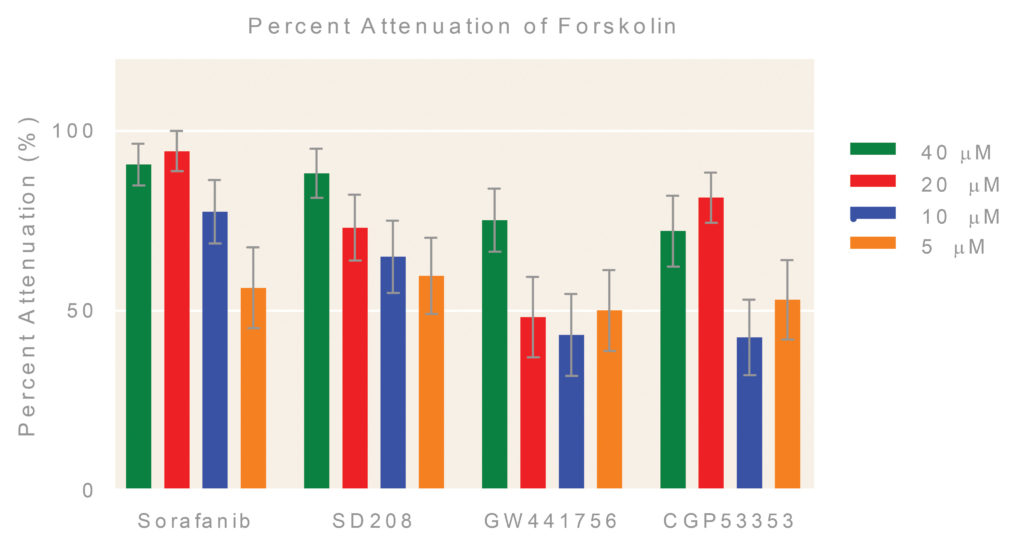
Selected hits: Several follow-up hits were assessed at different concentrations following the same procedure described previously, except that final DMSO concentration was increased to 0.9% (Figures 5 & 6).

Conclusions
- Corning Matrigel matrix 3D plates provide an easy solution
for forming 3D, polarized structures without the need for
self-coated plates. - The 384-well format makes Corning Matrigel matrix 3D plates
suitable for high-throughput applications. - Corning Matrigel matrix 3D plates are amenable to imaging
applications.
Hilary Sherman is senior applications scientist and John Shyu, PhD, serves as scientific applications and support teams manager at Corning Life Sciences.


