November 1, 2012 (Vol. 32, No. 19)
New System Focuses on Intrinsic Amide Bonds
Standard methods for protein quantitation rely on colorimetric assays like those involving protein-copper chelation (BCA and Lowry assays) and dye-binding based detection (Bradford and 660 Assay) or ultraviolet (UV) spectroscopy.
While colorimetric assays are easy to use, they are highly sensitive to sample components (detergents and reducing agents), protein composition and structure, and dye-binding properties. In these assays, protein concentration is determined by comparing signals from samples of unknown concentration to signals from reference standards (composed of common proteins such as bovine serum albumin), which are prepared for every measurement. Standard curve determinations differ considerably from assay to assay, affecting measurement reproducibility.
The BCA assay involves a two-step chemical reaction requiring incubation, and delivers results that can vary due to the kinetic rate of the reaction. In addition, phospholipids, chelating agents, reducing agents, and certain nonionic, oxidizing detergents can negatively impact the assay signal. The Bradford assay relies on binding of Coomassie Brilliant Blue G250 to basic amino acids, particularly arginine.
As a result, the assay outcome depends on the number of basic amino acid residues in the analyzed protein, which can vary greatly between proteins. Additionally, because the assay requires standard curve generation for every experiment, the proportion of basic amino acid residues in the protein standard needs to be similar to that of the protein being assayed.
UV spectroscopy-based quantification methods rely on the absorbance at 280 nm by a protein’s aromatic amino acids, predominantly tryptophan and tyrosine. Therefore, the absorbance at 280 nm of different proteins can vary widely (greater than twofold difference between extinction coefficients of albumin and immunoglobulin G). Additionally, proteins that do not contain aromatic amino acids, such as Protein A, cannot be quantified based on 280 nm absorbance.
Amino acid analysis delivers possibly the most accurate protein quantitation. The procedure consists of several steps—hydrolysis, derivatization, separation, and detection followed by quantitation. The results can be greatly influenced by variability in the inherent liability of a given sequence to hydrolysis, derivatization conditions, or sample contaminants (mainly presence of nonvolatile amines like Tris or glycine). Additionally, this method is expensive and may take time to obtain results if samples are sent to a third party for analysis.
Traditional Peptide Quantitation
Classical methods for peptide quantitation rely on the weight of the lyophilized powder, UV spectroscopy, or amino acid analysis.
Establishment of actual peptide concentration based on the weight of the lyophilized powder is inaccurate because the analyzed powder can contain a significant quantity (10−70%) of bound water, salts, or counterions. One UV spectroscopy-based peptide quantitation method relies on absorbance at 280 nm. This wavelength can be used to estimate peptide concentration if tryptophan and/or tyrosine residues are present in the sequence. Therefore, peptides that do not contain aromatic amino acids cannot be quantified using this method.
It is possible to determine peptide concentration by measuring absorbance at 205 nm. However, this measurement is far more prone to external influence, since many solvents and other chemicals will absorb at this wavelength. A high-quality, dual-beam spectrophotometer is required in order to reduce the effects of nonspecific absorption and to measure low concentrations.
IR-Based Quantitation
The Direct Detect™ spectrometer (EMD Millipore), an infrared (IR)-based protein quantitation system, represents an innovation in biomolecule quantitation. The key to this advance lies in the membrane technology for preparing and presenting aqueous biological samples to make them compatible with infrared analysis. The technology employs a hydrophilic polytetrafluoroethylene (PTFE) membrane that is spectrally “transparent” in the amide I and II regions used for protein and peptide detection and quantitation, enabling application of biomolecule solutions directly onto the membrane without added sample preparation.
By measuring amide bonds in protein and peptide chains, the system accurately determines an intrinsic component of every protein and peptide without relying on amino acid composition, dye binding properties, reaction kinetics, or redox potentials.
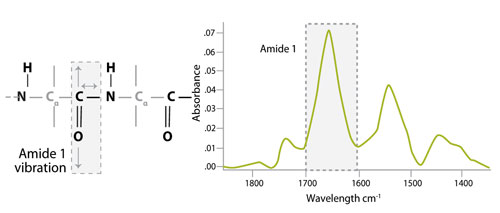
IR spectroscopy exploits the fact that molecules absorb radiation at specific frequencies characteristic of their structure and functional groups. Amide bonds within the protein absorb radiation in multiple regions of the IR spectrum, including a strong band at 1,600−1,690 cm-1 (amide I). In order to determine protein concentration, the Direct Detect system uses the intensity of the amide I band, which is assigned to C=O stretching vibration of the peptide bond (about 80%) with a minor contribution from C-N stretching vibration (about 20%), as shown in Figure 1. A key advantage to this system is that IR-based amide bond quantitation is not subject to interference from detergents, reducing agents, and chelators.
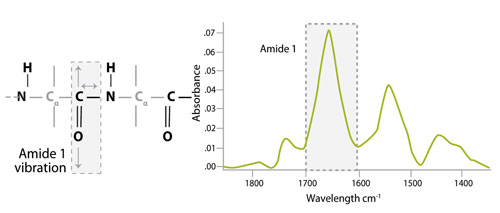
Figure 1. Vibrations responsible for the amide I band in the infrared spectra of proteins and peptides.
Standard Curve Generation
Assay-free, IR-based protein and peptide quantitation involves measuring intensity of the amide I band in the protein/peptide spectrum and subtracting the signal contributed by the buffer alone. The resulting amide I signal strength is then converted to the concentration by comparing it to the standard curve. The instrument requires a standard curve to be generated only once. Results obtained on 21 systems preloaded with the same standard curve showed highly reproducible protein quantitation (4.23% CV; data not shown). Benchmarking every experiment to the same, robust standard curve provides more reproducible results and facilitates intra-assay comparison across multiple experiments.
Dynamic Range, Accuracy, and Reproducibility
The system has been optimized for protein and peptide quantitation within the range 0.25−5 mg/mL. The accuracy of concentration estimation within the instrument dynamic range was established using single protein solutions, protein mixtures, and peptide solutions. Resulting concentration determinations were comparable to the data from amino acid analysis.
In contrast to other currently available methods estimating protein concentration, the Direct Detect system allows the user to perform multiple measurements without additional sample requirement. The assay-free sample card permits multiple analyses of the same sample that can be separated by hours or days.
Repeated measurements of the assay-free card show as little as 1.5% error on a single instrument and 2.6% error across multiple units (data not shown).
The accuracy of quantitation is not affected by the presence of reducing agents and detergents. Figure 2 compares performance of colorimetric assays and IR-based protein quantification in presence of additives. While concentration determination obtained on the Direct Detect system matches the standards regardless of measurement conditions (A), the Coomassie Plus (Bradford) assay results are affected by the presence of 1% sodium dodecyl sulfate (SDS) (B), and the Micro BCA™ assay could not provide any reliable concentration determination in the presence of 50 mM dithiothreitol (DTT) (C).
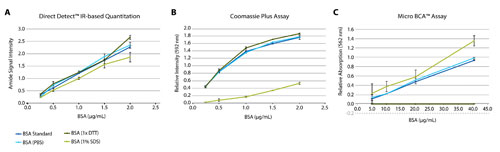
Figure 2. IR-based quantitation provides accurate and precise results, even in presence of detergent (SDS) and reducing agent (DTT).
A sample spectrum (Figure 3) shows that SDS does not have a strong signal in the amide I region and explains why SDS does not interfere with IR-based protein quantitation. Similarly, the IR spectra of reducing agents such as DTT or β-mercaptoethanol do not interfere with amide I quantitation (spectra not shown).
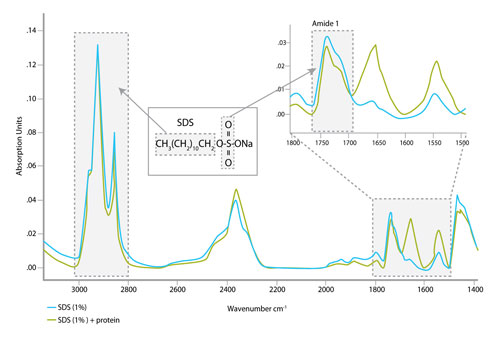
Figure 3. The characteristic peaks of the SDS IR spectrum are distinct from the amide I region of the protein spectrum.
Technology Features
IR-based protein and peptide quantitation relies on the vibrations characteristic for the amide bond, a chemical structure that is not uniquely reserved to protein and peptides only. Amides are present in other biomolecules, such as nucleic acids. Therefore, pure DNA or RNA can be quantified using the amide I region of the IR spectrum.
Interestingly, the amide vibration characteristic to nucleic acids is much weaker than the one observed for proteins. Additionally, amounts of nucleic acids present in most lysates are below the instrument’s detection level, allowing accurate protein quantitation.
Some commonly used buffers (e.g., urea) or buffer components contain amides, making these sample components incompatible with IR-based detection. At the same time, IR spectra deliver much more information about the analyzed sample than just the amide signal. As discussed, one key advantage of the Direct Detect system is that it accurately quantifies proteins in the presence of reducing agents and detergents, delivering spectral information on the type of additive present in the sample.
For example, Figure 3 shows IR bands characteristic for SDS: a strong signal between 2,800 and 3,000 cm-1 contributed by vibrations of the aliphatic groups (CH2 and CH3) and a weaker band at around 1,725 cm-1 assigned to S=O bond. Similar information can be collected for other buffer components, leading to robust databases that would allow identification of unknown buffer additives.
In conclusion, the Direct Detect IR-based quantification system provides a new way of accurately quantifying proteins and peptides based on their intrinsic amide bonds. The technology provides a highly reproducible, direct, assay-free measurement that is compatible with cell lysates and many common detergents and reducing agents. The spectra can also provide additional useful information about the sample composition.
Ivona Strug, Ph.D. ([email protected]), is senior biochemical scientist, Christopher Utzat, Ph.D., is research scientist III, and Timothy Nadler, Ph.D., is senior R&D manager at EMD Millipore.