March 15, 2014 (Vol. 34, No. 6)
Assessing Surface Technology for Informed Microplate Selection
There is an extensive selection of different microplates currently available, designed to offer a high degree of flexibility and a wide range of characteristics to suit different applications in basic research and diagnostics. To make the best use of the different plates available, researchers need to carefully choose the most suitable plate for their application. In this tutorial, we provide an overview of the types of plates available and the applications to which they are best suited, so that you can be confident that you will choose the best plate for your assays.
Performing accurate and reproducible assays in research and diagnostics requires careful reagent optimization and error-free liquid handling. Equally important is the selection of the ideal assay plate type for any one particular assay. The variety available continues to grow, driven in part by the desire to adapt more biological assays to suit a microplate format, with the aim of enabling larger numbers of samples to be processed, often via easier integration with automated workflows.
To render plates more effective for specific assay types, microplates can be optimized for parameters such as cell adhesion, chemical resistance, and compatibility with different methods of optic measurement. As such, a wide selection of plates exhibiting different colors, well shapes, and constituent materials is now available (Table 1).
Depending on the plate reader being used and the type of optical data that must be collected, plates can be specifically selected to optimize the accuracy of absorbance measurements at a given wavelength or the sensitivity for fluorescence or chemiluminescence measurements. For applications such as diagnostic tests, ELISAs, and color-end-point determination assays, simple 96-well microplates made of solid, clear polystyrene are adequate.
However, in vitro diagnostic (IVD) applications for quantitative and qualitative measurements of minute amounts of substances (such as hormones, viral and bacterial proteins, antibodies, binding peptides, and biomolecular disease markers) require high-quality assay plates with the appropriate surface to obtain accurate data, as is essential when attempting to precisely diagnose the presence or absence of patient disease.
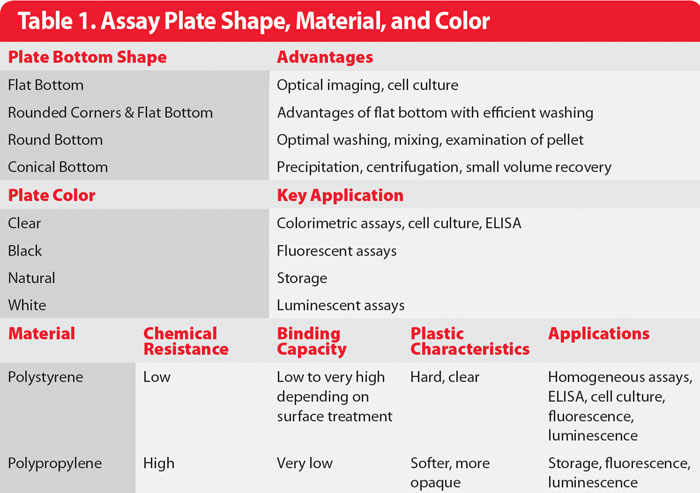
Table 1. Assay Plate Shape, Material, and Color
Why the Surface Characteristics of Plates Matter
When selecting your assay plate, a number of points need to be taken into consideration to ensure accurate and reproducible results, including the type of biomolecule being measured, plate surface characteristics and format, and intended plate storage conditions and shelf-life.
Microplates can exhibit chemical characteristics that promote interactions with biomolecules, whether favorable or undesired. For most applications, the interaction between the plate surface and biomolecules is exploited to optimize the coating of the microplate surface with the desired molecules.
This is the case for ELISAs, where molecules such as the antigens present in a sample are immobilized on the surface of the plate, either nonspecifically or via adsorption to the surface using antibodies or capture molecules. These are then detected using enzyme-linked antibodies specific to the antigen, via an enzymatic substrate that is converted into a detectable signal.
Effectively immobilizing specific biomolecules on an untreated plate surface can be a tedious process, while the amount of successfully immobilized molecules can vary greatly between plates, and even between the individual wells on a single plate. To ease this process and allow for quicker and more reproducible results, specific surface-treated plates can be chosen to match the characteristics of the biomolecule being immobilized.
One example of surface treatment is oxidation, which increases surface energy and hydrophilicity and is used for better cell adhesion and proliferation. This is also achieved via oxidized functional groups that provide highly effective coupling sites for the assay molecules of interest.
The interaction of biological components—whether cells, proteins, or phospholipids—with the plate’s surface can be the key to boosting assay performance. One particularly challenging assay type involves the formation of supported membranes (lipid bilayers) on the plate surface for measuring ion channel activity, transport across the membrane, or membrane fusion. Successfully carrying out such assays requires the even distribution of membrane attachment sites to enable the formation of a consistent lipid bilayer cover, with as few defects and holes as possible.
Knowledge of surface chemistry and the composition of functional groups on specialized surfaces is essential for choosing and optimizing the adsorption of assay components. As indicated in Figure 1, using a plate specifically optimized for oligonucleotide binding can increase the efficiency of attachment in a predictable way.
There are three principal surface types used for biomolecule attachment—passive adsorption, covalent binding, and affinity capture surfaces. Each surface type serves a different set of application requirements.
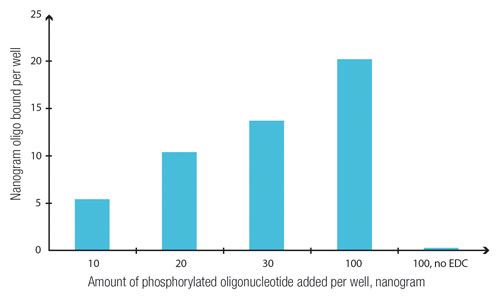
Figure 1. Amount of radioactively labeled oligonucleotides that bound to the plate surface (NucleoLink, Nunc™) with the help of ECD to cross-link protein to nucleic acid as a function of the amount added. (EDC: 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide.)
Passive Adsorption Surfaces
Passive adsorption surfaces have a broad range of applications as they are capable of binding a variety of biomolecules with good residual activity. They work by forming stable bonds via multiple weak molecular interactions that also allow for different molecular orientations. As such, they are suitable for immobilizing medium to large molecules, including antibodies. The sites of molecular interaction are determined by the matching properties of the polymer surface and the biomolecule.
Passive surfaces can be further subdivided into four groups based on the degree of hydrophilicity of the assay plate surface:
- Very hydrophilic plates offer high-affinity binding of most hydrophilic proteins and show increased binding sensitivity to pH.
- Standard hydrophilic plates, just one step down in hydrophilicity, can bind many biomolecules, both hydrophobic and hydrophilic, and are ideal for binding high concentrations of polyclonal antibodies and for antibody sandwich assays such as ELISAs.
- Slightly hydrophilic plates suit a diverse range of biomolecules including glycoproteins, serum-containing samples, and amphoteric molecules (such as lipopolysaccharides).
- Hydrophobic plates are used for the adsorption of highly hydrophobic molecules such as those that are lipid-rich.
Advice for selecting passive surface plates can be found in Table 2.
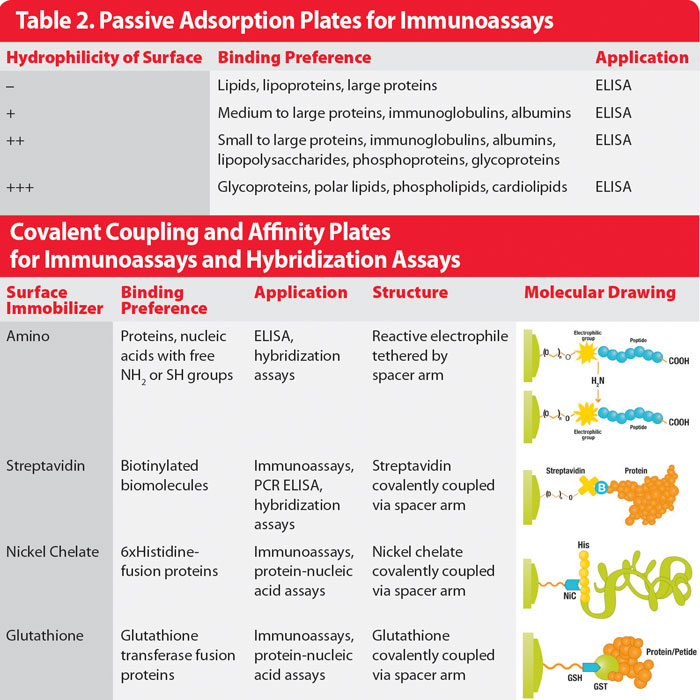
Table 2. Passive Adsorption Plates for Immunoassays
Covalent Binding Surfaces
The second type of plate surface, covalent binding surfaces, is designed to enable the formation of a single covalent bond between the polymer surface and the biomolecule. Such covalent surfaces are ideal for biomolecules of any size, as long as they contain the appropriate functional groups to enable covalent bonding. These reactive groups include free carboxyl and phosphate groups (electrophilic groups), which enable the effective binding of peptides, haptens, and oligonucleotides.
For example, peptides can be linked via a free primary amino or sulfhydryl group which bonds with electrophilic groups on the plate surface. This chemistry boosts assay sensitivity by reducing nonspecific binding, while also increasing speed by eliminating the need for time-consuming blocking steps.
As bonds can only form between these functional groups and the surface, the user can effectively predict and manipulate the molecular orientation of binding. Spacer arms can also be used to increase accessibility and thereby surface reactivity. Lastly, covalent bonds are strong molecular interactions, making these plates compatible with vigorous washing steps.
Affinity Capture Surfaces
The third surface type offers highly specific binding thanks to the use of specific receptor molecules immobilized on the plate surface. These high-affinity capture surfaces bind to tagged biomolecules via very specific interactions that reduce nonspecific binding, ensure consistent molecular orientation, and reduce background.
Widely used receptor-tag pairs include streptavidin-biotin, nickel-polyhistidine, and glutathione-GST peptide. Streptavidin can be passively coated onto the plate or covalently bound via a spacer arm to enhance precision. Nickel chelate groups and GST peptides are generally attached to the plate surface via a spacer arm. All can be used to facilitate the binding of a wide range of biomolecules. See Table 2 for advice on selecting affinity capture plates.
Conclusion
In summary, assay microplates are available in a bewildering variety of plate types, many of which have physical or chemical modifications on their surfaces to optimize them for specific applications. As such, the careful selection of plate type can significantly improve assay performance, accuracy, and reproducibility.
To facilitate the selection of the most suitable plate, several companies offer online or printed plate selection guides that can provide researchers with a detailed overview of the plate types, surface treatments, and applications available. By making an educated decision about which plate type to use before they undertake their experiments, researchers can increase the efficiency of their work and the accuracy of their results.
Thomas Andersen ([email protected]) is a senior technical adviser for laboratory specialty products at Thermo Fisher Scientific.


