Neuronal cell cultures have been successfully used throughout neuroscience research for decades. They provide scientists with dynamic and convenient model systems to study basic neuronal function and morphology, model disease, advance drug development, and evaluate neurotoxicity.
Consequently, the abilities to establish and maintain primary neuronal cultures and to keep neurons alive and functioning as they do in the brain for extended periods are essential for the comprehensive study of neuronal development and function. Neurons are extremely sensitive to being taken out of their natural environment because neurons and their intricate extensions and extensive connections work in complex microenvironments.
In addition, consistently obtaining viable neurons for experiments can be difficult, primarily due to limited neuron cell longevity and the inability to maintain cell health and functional integrity for the entire cellular life. These challenges can limit the sustained and in-depth studies that help researchers fully understand brain function.
When existing media systems are used, primary rodent neurons typically survive only for around 14 days in vitro, and their health degenerates significantly during that period. Functional in vitro studies of neurons and neural networks require that the cells are maintained for prolonged periods of time and at an optimal density.
For primary rodent neurons, full functionality and maturation requires maintenance in vitro for approximately 21 days. Thus, as the desire for more reliable and biologically relevant models has increased, so too has the necessity for a next-generation media system that can maintain and mature optimal densities of functional neurons over longer periods of time in the dish.
This tutorial describes how Thermo Fisher Scientific’s legacy Gibco B-27 Supplement and Neurobasal Medium was improved to create the B-27 Plus Neuronal Culture System. The new system enables neuroscience researchers to experiment for longer periods of time with neurons that reside in a dish, look and function like neurons in the brain, and resist neuron degeneration.
Optimized for Neuronal Cultures
Specific improvements made to B-27 included optimized formulation, upgraded manufacturing processes, and more stringent quality control for raw materials and final product. The new system is specifically optimized for the maturation and viability of primary rat, mouse, and human pluripotent stem cell–derived neurons for long-term cultures at both low and high cell densities.
An independent evaluation by scientists at Axion BioSystems used the company’s Maestro Pro microelectrode array (MEA) platform to verify the B-27 Plus system across criteria, including neurite outgrowth, neuron viability, and relative purity and functionality. Cultures were assessed at 7, 14, and 21 days following the plating of primary neurons or the addition of maturation medium to human pluripotent stem cell–derived neurons. The cultures were then assessed for cell survival and neurite outgrowth by quantitative morphometric analysis using Essen BioScience’s IncuCyte Zoom Live-Cell Analysis System.
Neuronal cell numbers (viability over time) were assessed by quantitative immunocytochemistry using HuC/HuD staining to demarcate neuronal populations. HuC/HuD staining is specific to neuronal cell bodies, facilitating quantification and throughput using the automated high-content analysis capabilities of IncuCyte Zoom.
In addition, primary rat neurons cultured in the Neurobasal Plus and the B-27 systems were also evaluated for synaptic maturity by quantitative immunocytochemistry of the synaptic markers synapsin and MAP2.
Major Improvements
The B-27 Plus system improved neuronal survival, neurite outgrowth, and neuron functionality and maturation.
Neuronal Survival
The rate of neuronal survival increased by more than 50%, reportedly the highest rate of in vitro survival of primary rodent and human pluripotent stem cell–derived neurons achieved to date. Reliable assessment of network electrophysiology requires measurements from many neurons in a network. The B-27 Plus system activated ≥15 electrodes out of a possible 16 electrodes per well in a 48-well MEA plate, the highest of all media tested. The number of active electrodes was also stable over more than 20 days in culture.
Figure 1 shows that B-27 Plus system maintained higher neuronal survival rates than did the B-27 system. Primary rat cortex neurons were cultured for up to three weeks in the listed media system and then immunostained for MAP2 on days 7, 14, and 21.
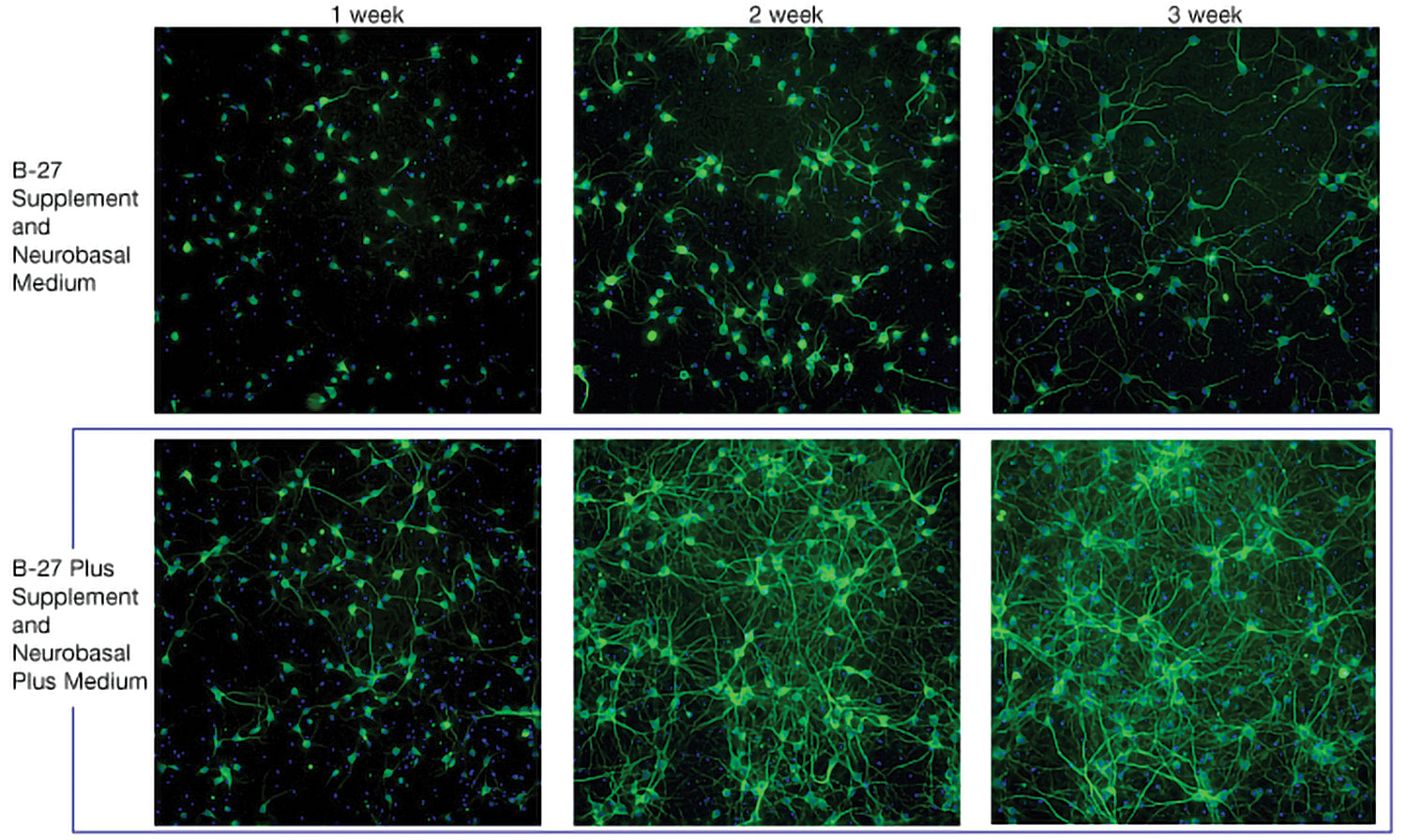
Prior to using the B-27 Plus System, the length of research studies using mouse primary hippocampal neurons would be a maximum of 14 days, as the neurons would begin to show reduced activity and viability by that stage. With B-27 Plus, scientists cultured mouse primary hippocampal neurons over a period of 28 days with ease, after which the viability of these neurons was tested by immunofluorescence and healthy morphologies; little to no cell death was observed.
Furthermore, the cells began to form more mature neuronal circuits, as evidenced by the large number of synapses and dendritic spines, equating to an accurate representation of in vivo condition, but in vitro.
Neurite Outgrowth
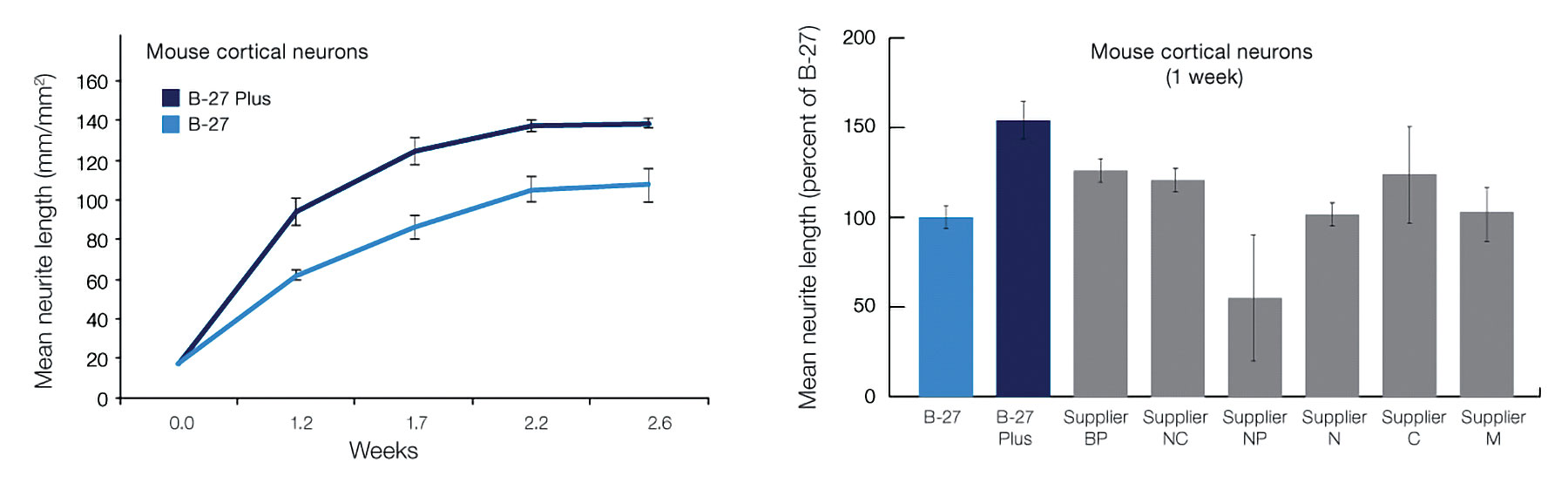
The B-27 Plus system significantly accelerates neurite outgrowth in short-term neuronal cultures and improves the electrical activity in long-term cultures relative to the classic B-27 system. Plates were scanned at various time intervals using IncuCyte Zoom. Neurite length was quantitated from phase-contrast images by applying the IncuCyte NeuroTrack neurite analysis tool.
Primary mouse cortical neurons were thawed in classic Neurobasal Medium with B-27 Supplement and plated onto poly-d-lysine-coated 96-well plates. Neurons were maintained for about three weeks in Neurobasal Medium with B-27 Supplement, Neurobasal Plus Medium with B-27 Plus Supplement, and other commercially available serum-free neuronal media systems following the suppliers’ recommended protocols.
Neurite outgrowth was quantitated using differential interference contrast images taken at the time points specified. The B-27 Plus system significantly accelerates neurite outgrowth over the first few weeks relative to the classic B-27 system. The results shown in the right panel of Figure 2 are from one of three experiments, with each run showing after one week that B-27 Plus enabled the highest mean neurite length relative to all alternative systems.
Neuron Functionality and Maturation
Synchronized network activity arises from numerous synaptic connections between the neurons in a mature network. The B-27 Plus system facilitated stronger network development, as demonstrated by the higher degree of synchrony seen with B-27 Plus, relative to that seen with other tested media systems.
Increased neural activity was observed using the B-27 Plus system. Primary rat cortex neurons seeded on CytoView MEA 48-well plates from Axion Biosystems (n = 3) were maintained for 35 days in vitro, with half-media changes every two to three days. Recordings were acquired 24 hours after media changes using the Maestro Pro MEA platform and then analyzed using the Axis Navigator software.
Different media types produced distinct patterns of neural activity. Qualitatively, the B-27 Plus system exhibited neural coverage across the entire array and a strong, synchronized network phenotype.
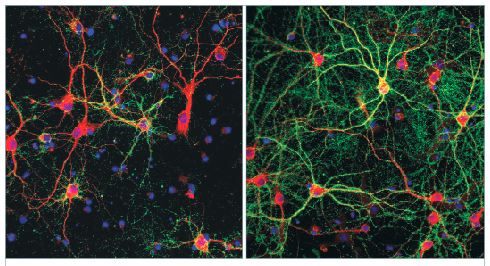
Figure 3 shows that neuronal maturation achieved using the B-27 Plus system is enhanced compared with that achieved using the classic B-27 system. Primary rat cortex neurons at day 22 were stained for the dendritic marker MAP2 (red) and Synapsin 1/2 (green) to label presynaptic terminals. Neurons maintained in the B-27 Plus system had significantly higher synaptic-positive puncta.
The B-27 Plus system, relative to the other tested media, promoted a higher degree of firing activity as the network matured.
Conclusion
The observed improvements—better in vitro survival rates and prolonged neuron health—lead to more economical production of the same number of neurons when compared to other products with no changes to current workflows. These improvements also allow superior modeling of neurons and advance new kinds of tests, including evaluations of whether drug candidates have therapeutic potential against neurodegenerative diseases.
The ability to perform long-term in vitro studies that recapitulate cellular structures and connections found in the brain is fundamental to advancing scientific understanding of the brain and neurological disease. This capability can lead to successful downstream applications utilizing these cells.


