May 15, 2007 (Vol. 27, No. 10)
Cell Painting and Cell Impermeant Dye-based Assay Distinguish Effector from Target Cells
One of the functions of the immune system is to recognize and destroy targets. This is accomplished by effector cells that employ cell-mediated cytotoxicity (CMC), natural killer (NK) activity, or antibody-dependent cellular cytotoxicity. The Guava® Cell Toxicity Assay (www.guavatechnologies.com) uses a cell painting dye and a cell impermeant DNA-binding dye to distinguish effector from target cells as well as to distinguish dead from live target cells.
The cell painting dye, CFSE, diffuses freely into cells where it is converted into a fluorescent, membrane-impermeant dye that is retained in the cytoplasm even after cell death. Painted target cells are incubated with unpainted effector cells. The dead-cell dye, 7-AAD, then identifies which of the painted target cells have been killed. The Guava Cell Toxicity Assay works on all Guava instruments.

Figure 1
Materials & Methods
NK Experiments. Nonadherent human bone marrow (K562) and human B-cell (Daudi) cell lines were kept in log phase growth in complete medium. Peripheral blood mononuclear cells (PBMCs) from anonymous healthy adult donors were obtained by gradient centrifugation over ficoll gradient, and NK cells were purified from them using the NK Cell Isolation Kit II according to manufacturer’s instructions. NK cells were typically >90% pure. Cell lines were seeded two days prior to each experiment. K562 cells were split to 300,000 cells/mL and Daudi cells to 400,000 cells/mL, incubated with 5 µM CFSE, and washed. For samples to be acquired on a traditional flow cytometer, target cells were painted with a lower concentration of CFSE. Painted target cells were mixed with NK cells at various effector-to-target ratios, including target cells only and effector cells only, in round bottom 96-well plates and incubated for 4 hours in a 37°C humidified 5% CO2 incubator. In some experiments, Interleukin-2 (IL-2) was added along with the effectors and targets prior to incubation. After incubation, 7-AAD was added to all wells. Samples were then acquired on a Guava PCA, Guava PCA-96, or Guava EasyCyte™ Microcapillary Flow Cytometry System.
CMC Experiments. PBMCs were obtained by gradient centrifugation over Histopaque Plus on day-old blood from two donors. Half of the PBMCs from donor A were frozen in supplemented RPMI. The second half were incubated with 25 µg/mL of mitomycin C in supplemented RPMI in a 37°C humidified 5% CO2 incubator for 30 minutes and then washed with supplemented RPMI and mixed at a 1:1 ratio with responder cells. The cell concentration of the mixture was adjusted to 1 x 106 cells/mL and incubated in a 37°C humidified 5% CO2 incubator for six days. On day 5, the frozen PBMCs were thawed, resuspended in supplemented RPMI, and incubated overnight in a 37°C humidified 5% CO2 incubator. The thawed PBMCs (targets) were incubated with 5 µM CFSE and washed. The effector cells (responders from donor B plus mitomycin C-treated stimulators from donor A cultured for a week) were washed once and resuspended. The painted target cells were mixed with effector cells at various effector-to-target ratios in round bottom 96-well plates and incubated for 4 hours in a 37 °C humidified 5% CO2 incubator. After incubation, 7-AAD was added to all wells. Samples were acquired on a Guava PCA-96 System.
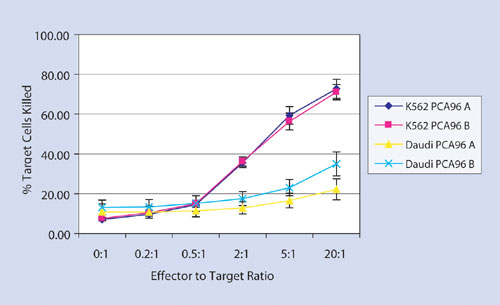
Figure 2
Results
Figure 1 shows typical results for NK assays. The dot plot shows a clear distinction between live and dead target cells, and a clear separation between effector and target cells. In this example, purified NK effectors mixed with painted K562 cells at a 1:1 ratio resulted in approximately 50% killing of the target cells.
Purified NK cells and CFSE-painted K562 cells were mixed at various effector-to-target ratios, incubated, and stained with 7-AAD. Results (data not shown) from the Guava Cell Toxicity Assay run on Guava PCA-96 and Guava EasyCyte Systems were comparable to results obtained with other similar assays and/or flow cytometry systems.
Daudi cells are known to be more resistant to NK activity than K562 cells. Figure 2 illustrates that the Guava Cell Toxicity Assay accurately reflects that difference in NK susceptibility, showing that many more K562 than Daudi cells were killed. Moreover, results were highly reproducible on two different Guava PCA-96 Systems.
Different mixtures of effector-to-target ratios were prepared using NK and K562 cells in 96-well plates. No bias of results over time or well location were observed, and the percentages of target cells killed for all samples were consistent over the time needed to acquire an entire plate. The %CVs for percent of target cells killed were under 10% at effector-to-target ratios that resulted in killing above background. Also, the %CVs for the MFIs were typically under 10%, showing that the populations stayed consistent over the plate.
Painted K562 cells were mixed with purified NK cells at various effector-to-target ratios and acquired at different times. The percent target cells killed and MFI values were similar for up to 24 hours for all ratios tested, indicating that samples kept at room temperature were stable up to 24 hours post-incubation.
Painted K562 cells were mixed with purified NK cells at various effector-to-target ratios and incubated in various vessels (i.e., microcentrifuge tubes and round- and flat-bottom 96-well plates). The most optimal NK activity was observed in round-bottom 96-well plates (data not shown). K562 cells were painted and mixed with purified NK cells at both a constant cell concentration and constant target concentrations, stained following the above protocol, and run on a Guava PCA-96 System. Identical results were obtained regardless of whether a constant total cell concentration or a constant target cell concentration was used.
Allogenically stimulated effector PBMCs were mixed with target cells from the same donor at various ratios and run on a Guava PCA-96 System. Figure 3 shows that the Guava Cell Toxicity Assay detected CMC, but, as expected, higher effector-to-target ratios were necessary than for the NK experiments.
IL-2 is known to induce a dose-dependent increase in NK activity. Painted K562 cells were mixed with purified NK cells and IL-2 was added at increasing concentrations. Results demonstrated that even at the lowest concentration, IL-2 caused a large increase in NK-mediated killing.

Figure 3
Conclusions
The Guava Cell Toxicity Assay was shown to:
• enable easy sample preparation, permitting completion of the assay in one day;
• distinguish between live and dead target cells in a single sample;
• differentiate dead effector from dead target cells, decreasing nonspecific background;
• automatically calculate and report percent target cells killed for each sample;
• provide cell concentration and percent total or gated for the four cell populations;
• accurately determine percent of killing at multiple effector-to-target ratios;
• precisely determine percent killing and MFI of target cells;
• provide stable samples for up to 24 hours post incubation;
• work for both NK and CMC assays;
• detect IL-2-enhanced NK activity.
Lisa B. English, Ph.D., is in the
communications department and Katherine Gillis and Dianne Fishwild are in the R&D group at Guava Technologies.
Web: www.guavatechnologies.com.
E-mail: [email protected].


