August 1, 2008 (Vol. 28, No. 14)
Evaporative Light-Scattering Detectors Improve upon Refractive Index Systems
Since the first high performance liquid chromatographs (HPLC) hit laboratories in the 1960s, refractive index (RI) detectors have enabled the discovery of testing materials that do not possess ultraviolet absorbance. Through the years, however, researchers have determined that RI detectors do not always effectively detect target substances with short elution times. For example, RI detectors are unable to perform gradient analysis, and they are prone to giving negative peaks, making quantitative analysis difficult.
Twenty years later, a solution arrived for these problems when evaporative light scattering detectors (ELSD) became commercially available. ELSD is a powerful, high-speed technique for detecting if a test sample is less volatile than the mobile phase. Initially overshadowed by the significant advances in HPLC, today’s ELSDs offer a wide range of testing abilities that improve upon many of the disadvantages of RI detectors in specific applications.
The principle behind ELSD is simple. Target components are converted to a fine spray by a nebulizer and heated so that only the mobile phase is evaporated. Light is directed at the remaining target substances, and scattered light is detected. This makes ELSD an ideal detector for drug discovery, combinatorial chemistry, polymers, and the analysis of natural substances.
Comparing ELSD and RI Detectors
Similar to RI detectors, ELSDs are classified as universal detectors. ELSDs, however, differ from RI detectors in various ways. ELSDs are five to ten times more sensitive than an RI detector, they support the use of the gradient elution method, are not easily affected by changes in ambient temperature or interference due to solvent peaks, and do not require time for the instrument and baseline to stabilize.
The gradient elution method is effective for batch analysis of multiple components of natural products, but RI detectors can not be used for this testing because of changes in the baseline caused by changes in the RI of the mobile phase. With ELSD, baseline changes do not occur during gradient elution. This means that ELSD is ideal for efficient, high-sensitivity analysis of multiple components.
Testing with ELSD is useful for analyzing compounds that cannot be detected with UV detectors such as carbohydrates, sugar alcohols, surfactants, and natural and synthetic molecules. It is also preferred for the batch analysis of compounds where gradient elution is difficult because absorbance occurs only in the short wavelength region. These compounds include fats, glycerides, fatty acids, and phospholipids.
Additionally, ELSD can be applied to all methods used with liquid chromatography/mass spectrometry. Even in the mobile phase, scientists and researchers can use ELSD instead of expensive LC/MS instruments to screen compounds.
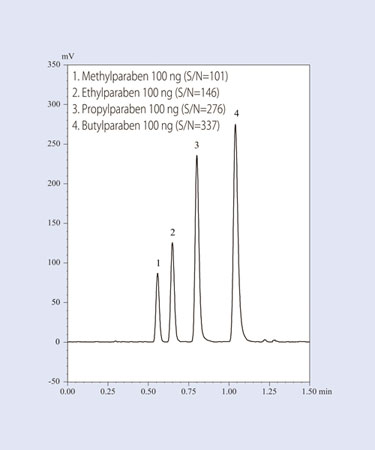
Figure 1
Inner Workings of ELSD
Although the design of ELSD varies from manufacturer to manufacturer, the testing process occurs through three successive steps: nebulization, evaporation, and optical detection.
Following separation in a column via HPLC, the target substance and its mobile phase are nebulized into a spray of tiny droplets using a gas stream. The spray is carried to a drift tube where heat is applied so that only the mobile phase evaporates. The remaining target substance from the mobile phase converts to minute solid particles, which are carried to the ELSD.
Once inside the detector, the target substance’s particles cause the light emitted from the light source to scatter. Scattered light created by the collision of light with the minute particles emerging from the drift tube is detected and measured by a photomultiplier. The intensity of the signal captured by ELSD can be detected with the following equation:
Signal intensity = A x (quantity of
target substance)B
In this formula, A and B are constants determined by several factors including particle size, concentration and type of target substances, gas flow rate, mobile-phase flow rate, and the drift tube’s temperature.
ELSDs can analyze substances that have evaporation temperatures lower than that of the mobile phase. They also can achieve about the same level of detection sensitivity for any compound. If the target substance is nonvolatile, detection is possible down to the nanogram level.

Figure 2
High-Sensitivity Detection
In ELSD, larger nebulized particles require higher temperatures to evaporate. When analyzed at lower temperatures, droplets that do not evaporate create a high level of noise during testing. Some newer models of ELSD have solved this problem by adding a glass cell to the instrument.
Following nebulization, droplets that leave the ELSD are carried by the gas stream through the glass cell and into the drift tube. Larger droplets stick to the side of the glass tube instead of following the gas stream. The extra liquid collects in a siphon tube and is discharged (split siphon method). Large droplets that contribute to noise are separated selectively, and smaller droplets are carried into the drift tube more efficiently.
Using the glass cell, ELSD makes it possible to suppress noise even at low evaporate temperatures. Mobile phases can be evaporated at low drift tube set temperatures in the range of 35ºC to 40ºC, so high-sensitivity analysis is possible for most compounds. Also with this technology, assisting gas is projected in a cylindrical shape centered on the drift tube outlet. This increases the concentration of the target substances to reach the detection unit and improves sensitivity.
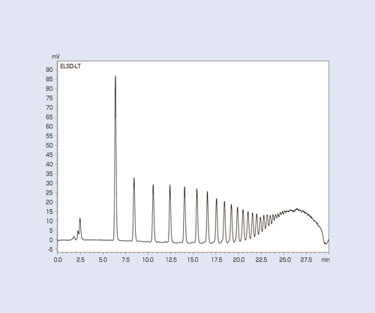
Figure 3
High-Speed Analysis
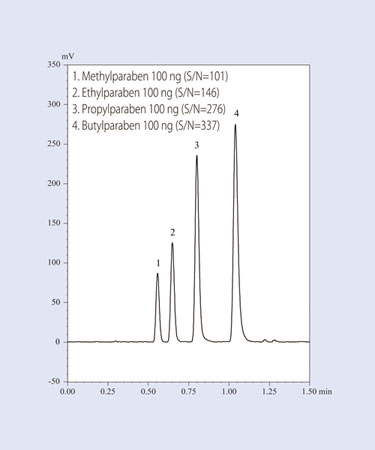
One of the greatest benefits of ELSD is its ability to support ultra high-speed analysis with HPLC (Figure 1). By pairing ELSD with HPLC, users can efficiently execute the batch analysis of multiple components in natural products.
Documented benefits of integrating ELSD and HPLC include: high-speed sampling rate (20 m/sec), suppression of peak spreading by reducing the drift tube’s inner diameter, and peak focusing using assisting gas.
The split siphon method ensures that only a fine mist enters the drift tube. The assisting gas focusing function guarantees that contamination of the detection unit is minimized. In this fashion, there is no loss of sensitivity, even when continuously analyzing multiple samples.
Applications
The standard method for analyzing amino acids by HPLC is either pre- or post-column derivation or detection by fluorescence because the acids lack suitable chromophores. This approach yields excellent sensitivity, but the derivatization process is time consuming and can introduce variability between each test run. ELSD excels at analyzing nonchromophoric compounds and eliminates the need for derivatization.
ELSD works by making a light-scattering measurement of target analytes that have been dried of mobile phase via evaporation. The detector produces stable baselines during gradient elution chromatography, and its response is independent of the spectral properties of the analyte and solvent.
Polyethylene glycol is difficult to analyze with a UV detector because it has little UV absorbance. Usually, an RI detector is used to analyze this compound. However, achieving separation with isocratic elution while using an RI detector is limited when separating low-molecular-weight polyethylene glycol to the polymerization level using a reversed-phase mode.
Figure 2 shows an example of results obtained when researchers subjected polyethylene glycol 1000 to gradient elution using the reverse-phase mode. Detection was performed with ELSD. The graph shows how using ELSD makes it possible to separate polyethylene glycol according to the polymerization degree with a stable baseline using the gradient elution method.
According to the glucose polymerization degree, the normal-phase mode demonstrates superior selectivity in the separation of oligosaccharides. However, the time needed for elution increases as the number of glucose units increases.
With an RI detector, gradient elution cannot be performed. So, analysis must be carried out repeatedly using mobile-phase compositions that are suitable for the separation of each polymerization degree. Using an ELSD makes it possible to identify maltooligosaccharides with glucose polymerization degrees of up to about 20. Figure 3 shows an example of results from subjecting maltooligosaccharides to gradient elution using the normal-phase mode.
Simon Robinson is HPLC product manager at Shimadzu Scientific Instruments. Web: www.shimadzu.com. Email: [email protected].