As the global population increases, traditional vaccine manufacturing models are coming under increasing scrutiny. They are struggling to satisfy rising demand and quality expectations for vaccines that are critical to preventing life-changing, and even fatal, infectious diseases. In the case of poliomyelitis (polio), which can cause debilitating paralysis or death, and for which there is no effective treatment available, the best strategy to protecting global health is prevention through immunization. Because traditional vaccine manufacturing platforms require high capital investment and offer low reimbursement levels, there have been limited efforts toward vaccine innovation in the industry over the past decade or more.
To bridge the gaps between the supply and demand for these life-saving vaccines and other biologics, Univercells has developed a novel manufacturing platform for flexible, reliable, and cost-effective production. The NevoLine™ system was first developed for the production of a trivalent inactivated polio vaccine, specifically, a Sabin strain–based inactivated polio vaccine (sIPV), with Vero cells at a final cost under $0.30/dose. This first application was performed under a grant from the Bill & Melinda Gates Foundation with consortium partners Batavia Biosciences and Natrix (Merck Millipore). The results of this study are illustrated in the case study that follows.
The NevoLine manufacturing platform combines intensified, continuous, and automated single-use bioprocessing approaches. In-line monitoring and controls contribute to cost-effective vaccines manufacturing with drastic CAPEX and footprint reductions. Designed with self-contained modules tailored to the biosafety level and bioburden control requirements (such as biosafety cabinets and isolators) for each process, the NevoLine platform is highly flexible for a range of applications, and for the implementation of flexible-facility concepts for rapid response to market demands (Figure 1).
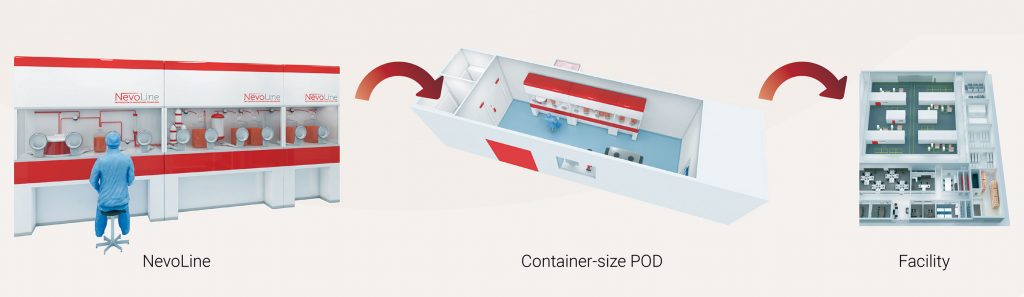
At the core of the NevoLine platform is the scale-XTM fixed-bed bioreactor, which is available in a wide range of configurations and provides up to 600 m² of area for cell growth within a 60-L bioreactor. The full platform supports the production of large quantities; in this case, over half a million doses per batch of sIPV. The NevoLine platform was designed to deliver high titers and high production yields while maintaining adequate product purity and quality. The economic benefits of the NevoLine platform for sIPV manufacturing are reviewed here.
sIPV vaccine manufacturing with the NevoLine platform has three stages (Figure 2): upstream processing (preculture, inoculation, cell culture, infection, and viral production); downstream processing (clarification, purification, and formulation); and inactivation processing. The Highly Intensified Process for sIPV (HIP-IPVTM) was developed by consortium partner Batavia Biosciences.
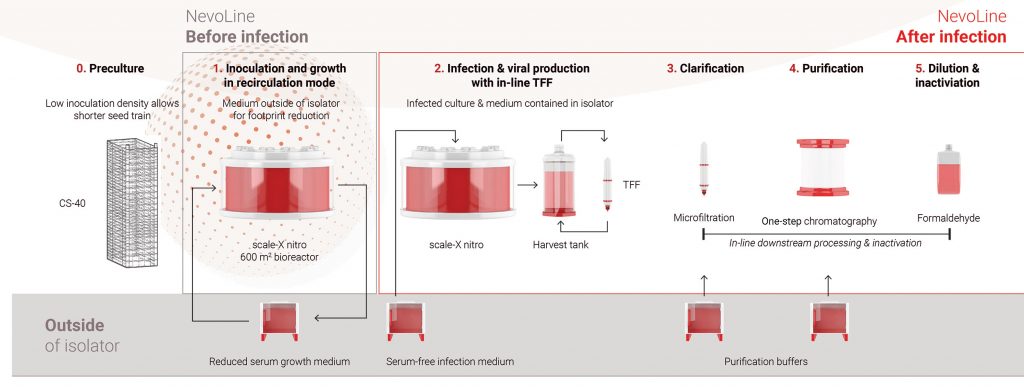
Equipment and consumables
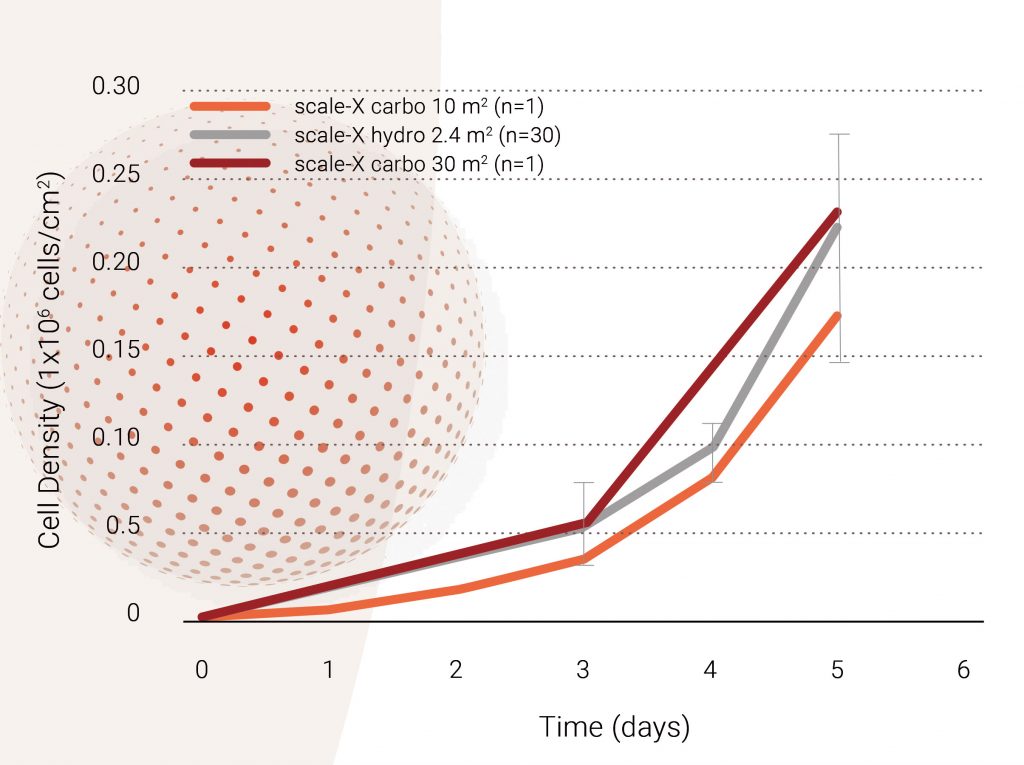
The process was developed at small scale (scale-X hydro bioreactor, 2.4 m² growth surface) and is being scaled-up to pilot scale (scale-X carbo bioreactor, 10–30 m² growth surface). To demonstrate further scalability, large-scale bioreactors will be used (scale-X nitro, up to 600 m² growth surface). See Figure 3.

Biological materials: Vero cells and Sabin attenuated polio strains. Assays: optical microscope for cell density monitoring via nuclei counts; TCID50, ELISA assay (D-antigen content), SDS-PAGE, HCP ELISA, and HCDNA qPCR for production and purity testing. Cost modeling: BioSolve 2017 (from Biopharm Services) used for cost comparisons at commercial (600 m²) scale. Reference process: traditional sIPV manufacturing with stirred-tank microcarrier process used for comparison.
Results for the sIPV production process
Cell growth in scale-X bioreactor: The growth profiles of the Vero cells in scale-X bioreactors at both small and pilot scales are shown in Figure 4. The use of intensified fixed-bed bioreactors with a high surface area per unit volume allowed for cell densities of up to 200,000 cells/cm² (or ~30 million cells/mL of fixed-bed) to be reached reproducibly within 5 days. A comparable growth profile was seen at a 2.4 m2, 10 m², and 30 m² scale.
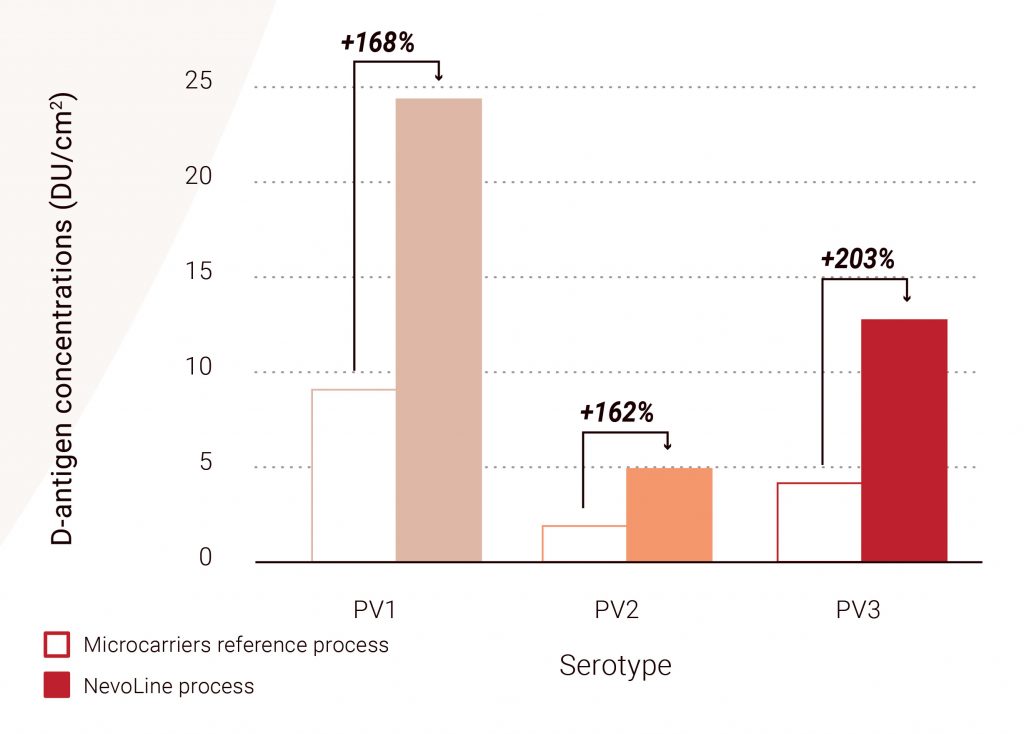
Viral expression: The D-antigen concentrations of the three serotypes of sIPV obtained at small scale are shown in Figure 5. The high titers achieved in scale-X bioreactor demonstrate a promising production potential compared to titers achieved in current microcarrier-based processes (up to +203% for PV3, n = 4). High viral titers were maintained during process scale-up to the 30 m² pilot scale (not shown here).
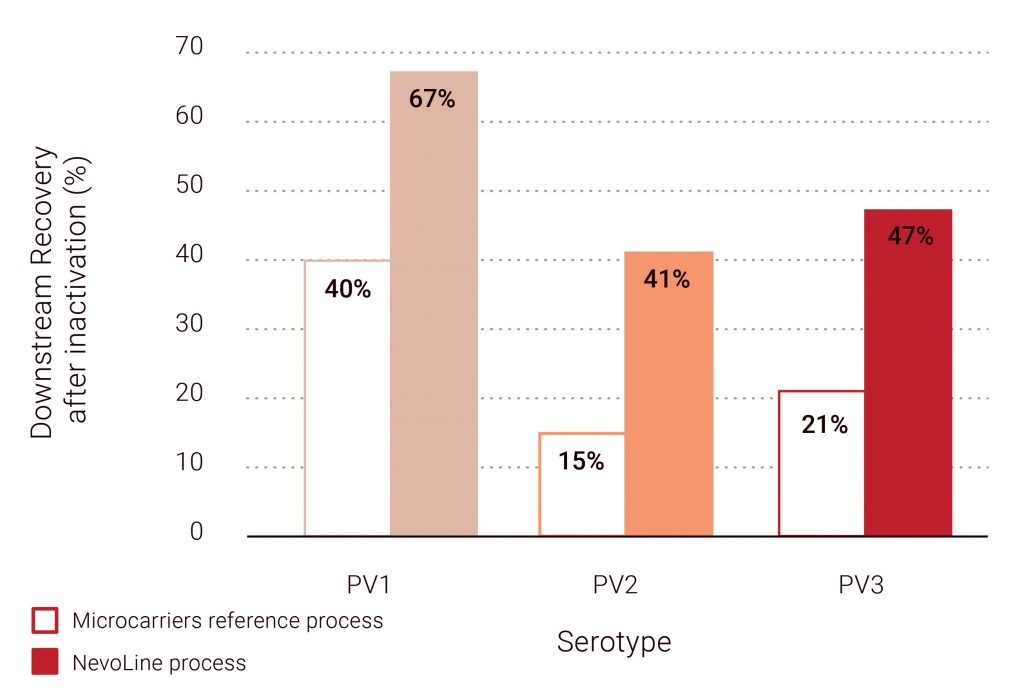
Optimized downstream processing: A one-step sIPV purification process was developed using a one-step capture chromatography column, resulting in a high purity and recovery. Process recoveries after inactivation are 1.6 to 2.7 times higher than in the reference microcarrier process, while achieving over 95% purity (taking whole cells and residual DNA into account, WHO specifications) (Figure 6).
Small footprint: The NevoLine platform was customized into three modules for upstream, downstream, and inactivation processes for sIPV production (Figure 7). Intensification of each unit step helped to drastically reduce the footprint of the equipment, which was contained in a series of isolators for increased safety. The final footprint covered 10 m². A highly automated interface limits the number of manual operations, and in-line decontamination guarantees safety.

Case study conclusions
The consortium led by Univercells successfully delivered a breakthrough low-cost manufacturing platform for polio vaccine. This case study demonstrates results under $0.30/dose for sIPV vaccine production, cutting the cost of production up to fivefold. Process and equipment scale-up to commercial capacity is the next challenge to make polio vaccines affordable and available to all.
Based on the successful first application for polio vaccine covered in this case study, it is demonstrated that the NevoLine platform is a flexible, cost-effective solution for global health viral vaccines for human and veterinary use; epidemic preparedness (low-cost stockpiling) and response strategies for outbreaks; and high capacity, cost-effective viral vector production for cell and gene therapies.
At Batavia Biosciences, Ahd Hamidi, PhD, is a bioprocess technologist working as head of Global Health Projects, and Christopher Yallop, PhD, serves as COO and CSO. At Univercells, Jean-Christophe Drugmand, PhD, is senior bioprocess architect, innovation and conceptual design, Andy Reniers is bioprocess engineer, Stephanie Dubois is program manager, and José Castillo ([email protected]) is company co-founder and CTO.

