April 15, 2018 (Vol. 38, No. 8)
Enabling High-Throughput Testing of Permeability and Cytotoxicity
The blood–brain barrier (BBB) is formed by the endothelial cells lining the cerebral microvasculature and tightly regulates passage of molecules from the blood into the brain. While necessary to help prevent harmful substances from reaching the brain, this defense system can also thwart entry of drugs intended to treat neurological conditions and brain cancer. Drug candidates for treating brain cancer must be assessed both for their ability to cross the BBB and also for their tumoricidal properties.
Traditionally, high-throughput testing of compound permeability through the BBB in vitro has been limited to the assay of radio- or fluorophore-labeled compounds as they pass a cell monolayer growing on a permeable support system. Unfortunately, the labels themselves may impact the assay, and the ability to determine resulting tumor cytotoxicity must be studied independently.
Evaluation of drug cytotoxicity has historically relied on use of cell monolayers, an approach that is less physiologically relevant as compared to three-dimensional (3D) cultures, which more closely mimic in vivo morphology, structural complexity, and phenotype.
Here, we describe a single 3D model to study both BBB transport and brain tumor cytotoxicity that combines Corning® 96-well spheroid microplates and the HTS Transwell®-96 tissue culture permeable supports. The combination of spheroid microplates and Transwells allows for a novel 3D model that can differentiate between compounds that can pass the BBB and those that cannot, while also assessing the resulting gliomasphere cytotoxicity.
Corning spheroid microplates are cell culture microplates with round well–bottom geometry coated with Corning Ultra-Low Attachment surface, enabling the formation of single multicellular tumor spheroids centered in each well in a highly reproducible manner. HTS Transwells are permeable supports commonly used for drug transport and migration/invasion studies. By replacing the standard flat-bottom Transwell receiver plate with the spheroid microplate, drug transport across the BBB and the resulting 3D glioma spheroid toxicity can be studied in an easy-to-use, 3D, high-throughput assay.
BBB Model and 3D Gliomasphere Formation
MDCKII/MDR1 cells were obtained from the Piet Borst Laboratory (Netherlands Cancer Institute) and were used as a BBB surrogate. The MDCKII/MDR1 cell line is derived from the kidney and transfected with the human MDR1 gene. When grown on Transwells, the cells differentiate into columnar epithelium and form tight junctions; they have been used extensively for P-glycoprotein (P-gp/MDR1)-mediated drug efflux studies.
Cells were seeded into HTS 96-well Transwell permeable supports (Corning Cat. No. 3391 or 3977) at 100,000 cells per cm2 in 100 μL of Dulbecco’s Modification of Eagle’s Medium (DMEM; Corning Cat. No. 10-013-CM) supplemented with 10% fetal bovine serum (FBS; Corning Cat. No. 35-010-CV). The cells were cultured for five days with a medium exchange 24 hours prior to assay. Monolayer integrity was assessed via Lucifer Yellow permeability (Sigma Cat. No. L0144) and rhodamine 123 P-glycoprotein (Pgp) efflux (Sigma Cat. No. R8004). Immunostaining of MDCKII/MDR1 monolayers was performed in order to confirm presence of tight junction proteins ZO1 (Thermo Fisher Cat. No. 339188) and occludin (Thermo Fisher Cat. No. 331588) per the manufacturer’s protocol.
MDCKII/MDR1 cells can successfully form a barrier on Corning HTS 96-well Transwells and are capable of demonstrating low Lucifer Yellow permeability, high Pgp efflux activity, and tight junction formation (data available for download).
LN229 human glioblastoma cells (ATCC® Cat. No. CRL-2611™) were routinely cultured in DMEM containing 10% FBS. Cells were harvested with Accutase® cell detachment solution (Corning Cat. No. 25-058-CI) and seeded into 96-well spheroid microplates at 1,000 cells per well for 24 hours prior to assay.

Figure 1. The combined BBB surrogate model and gliomasphere consisting of Transwell permeable supports and spheroid microplates.
BBB/Gliomasohere Model Test
Figure 1 provides a schematic of the combined BBB model and glioma spheroid (gliomasphere). After five days of BBB formation, medium from the Transwells was aspirated, and medium in the apical chamber was replaced with 250 μM of the chemotherapeutic agents cisplatin, piperlongumine, or buffer that had been matched to contain DMSO as the piperlongumine vehicle control. Inserts were then combined with 24-hour-old LN229 spheroids for 2 hours at 37 ºC. After 2 hours of co-incubation, inserts were removed and tested for barrier integrity via Lucifer Yellow. LN229 spheroids were cultured for two days and then assessed for viability with CellTiter-Glo® 3D (Promega Cat. No. G9683).
Figure 2 shows LN229 gliomasphere viability following exposure to 250 μM of cisplatin or piperlongumine with or without the BBB surrogate. In the presence of the BBB surrogate, the cytotoxic effect of cisplatin was blocked. In contrast, the effect of piperlongumine, which can cross the BBB, was similar whether the BBB surrogate was present or not.
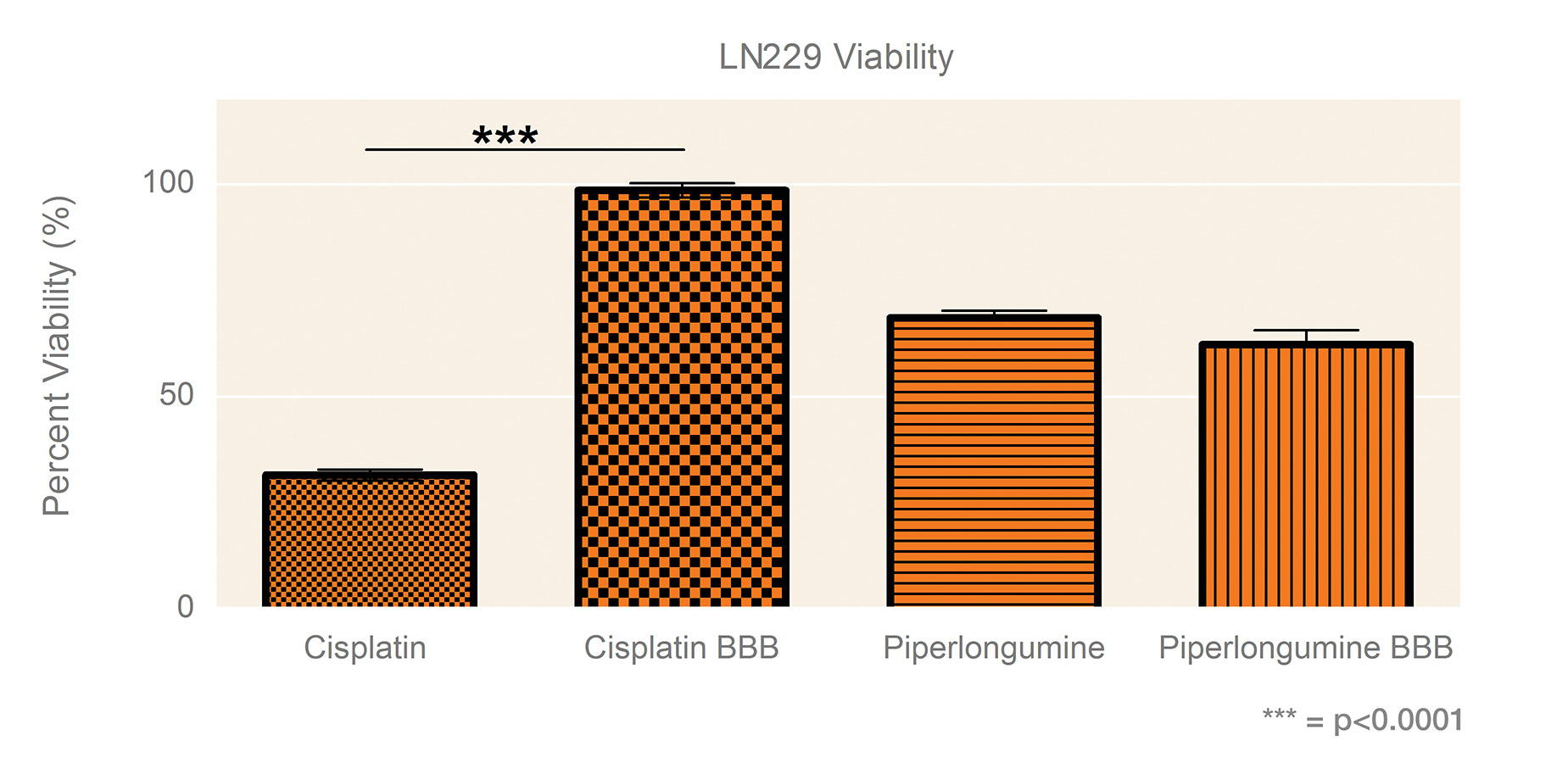
Figure 2. Percent viability of LN229 spheroids 48 hours following 2-hour 250 µM drug exposure through Transwell permeable supports with or without a BBB. Viability in buffer controls was normalized to 100%. Data shown as the average of three independent studies, N = 30. Significance was determined by one-way ANOVA with Bonferroni’s post-test.
Kinase Inhibitor Screening
After five days of BBB formation, medium from Transwells was aspirated, and medium in the apical chamber was replaced with 50 μL of compound from the Tocriscreen Kinase Inhibitor Toolbox (Tocris Cat. No. 3514), a collection of 80 kinase inhibitors supplied pre-dissolved in DMSO (250 µL 10 mM solution) or buffer. Inserts were then combined with 24-hour-old LN229 spheroids for 2 hours at 37 °C. After 2 hours of co-incubation, inserts were removed and tested for barrier integrity, and LN229 spheroids were cultured and assayed via CellTiter-Glo 3D.
Figure 3 shows the compilation of hits discovered with or without HTS 96-well Transwells containing the BBB surrogate. Inserts that exhibited higher than 10 nm/sec Lucifer Yellow permeability after compound exposure were discarded from analysis since monolayer was deemed compromised. Hits are defined as being 3 sigma below buffer response in at least two of three independent screens.
The results from this assay demonstrate that many of the hits that were identified without the BBB present are screened out when the BBB is present. This assay is more selective in identifying hits than assays missing the BBB component.
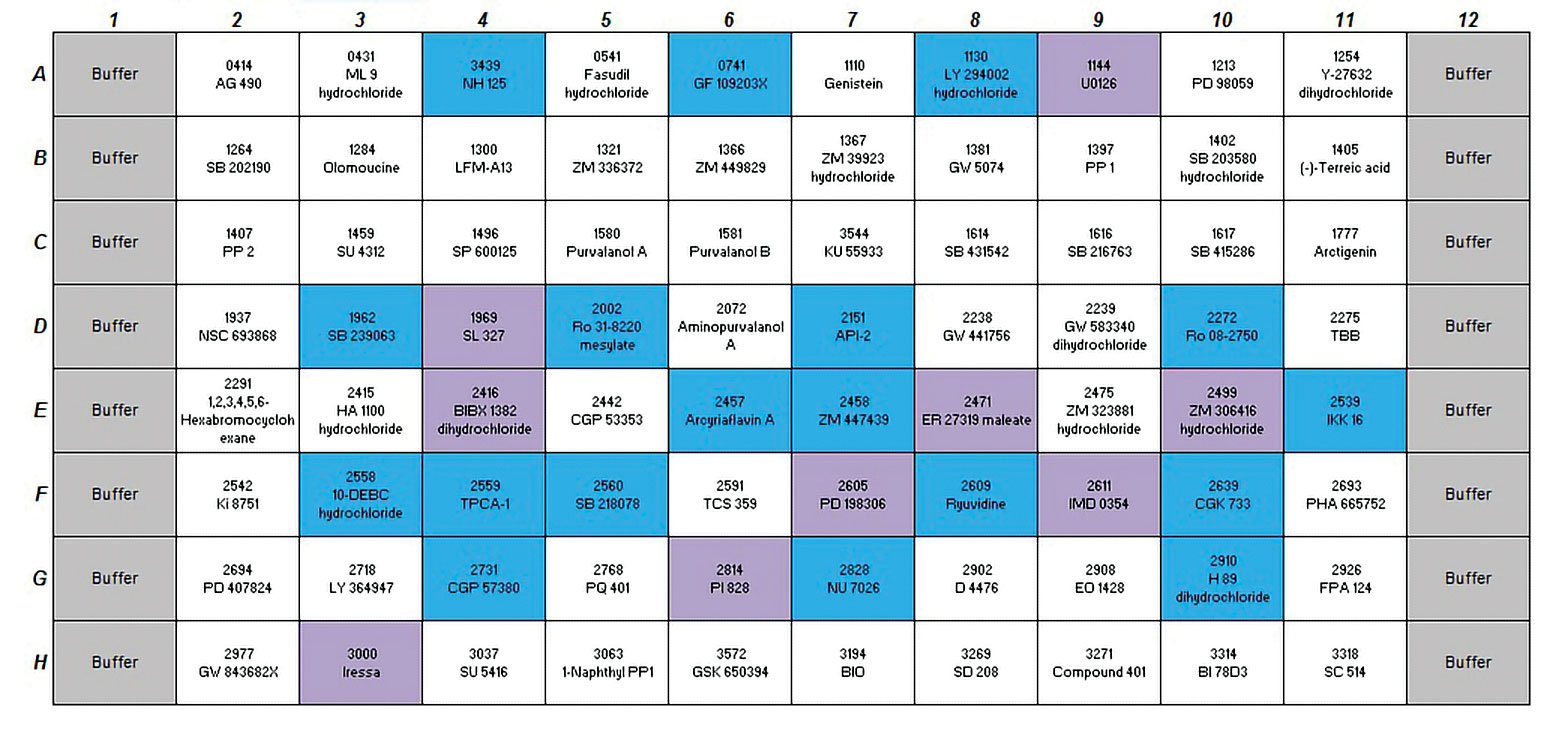
Figure 3. Effect of kinase inhibitors on gliomasphere viability as measured in 96-well spheroid microplates. Blue boxes indicate hits when BBB was absent. Purple boxes denote hits found both with and without BBB.
Combined Drug Transport and Cytotoxicity
The majority of cell-based screening is performed using 2D culture technologies, due in part to the demands of the automation and detection instrumentation in use. The shortcomings of 2D cultures for drug candidate screening have resulted in a growing interest in creating assays and cell environments that more authentically replicate in vivo conditions.
This tutorial described use of Corning spheroid microplates and HTS 96-well Transwell permeable supports to create an innovative, high-throughput, 3D BBB model to differentiate between compounds that can pass the BBB and those that cannot, while also assessing cytotoxic activity.
Specialized spheroid microplates have a round well–bottom geometry coated with Corning Ultra-Low Attachment surface. This design enables formation of consistently sized, single spheroids in each well that are ideally suited for drug screens. By replacing the standard flat-bottom Transwell receiver plate with the spheroid microplate, we achieve the ability to study drug transport across the BBB and the resulting 3D glioma spheroid toxicity in an easy-to-use, 3D, high-throughput assay.



