For Anima Biotech, it’s all in the translation—that is, mRNA translation. Drug development and small molecule discovery have long targeted small binding pockets or catalytic sites on proteins’ surfaces. This approach has yielded many therapeutic molecules as well as substantial returns on investment, but it is not without its pitfalls.

CEO, Anima Biotech
“That hasn’t changed significantly in the past 50 years,” says Yochi Slonim, CEO of Anima Biotech. “The problem with that approach is that small molecule drugs must have a binding site, but many proteins don’t have such pockets on their surface, which is why they are undruggable by this approach.”
Some highly conserved protein families are also notorious for having binding pockets so similar across the family that no small molecules have been found to specifically bind just one. “For instance,” Slonim explains, “the c-Myc protein is overexpressed in most known cancers, but conventional small molecule drugs can’t be designed to target it. Collagen, the most abundant protein in the human body, also is completely undruggable using conventional methods.”
Amina has found a workaround for these problems; the company bypasses the protein of interest altogether and target the translation of the mRNA for that protein—the biochemical equivalent of asking to speak to a manager. By targeting the translation of mRNA into protein on ribosomes to modulate the levels of protein produced, many previously undruggable targets become druggable. That means a new generation of small molecule drugs is being discovered with the potential to rival biologics not only in ease of administration, but also in ease of development.
Lighting the way
The company’s platform, Translational Control Therapeutics, combines a new biological technique, proprietary image analysis, and high-performance big data software. As Slonim says, “Instead of targeting proteins, we target the way they are created. Although the proteins differ from one another, ribosomes create them all by translating their mRNA.”
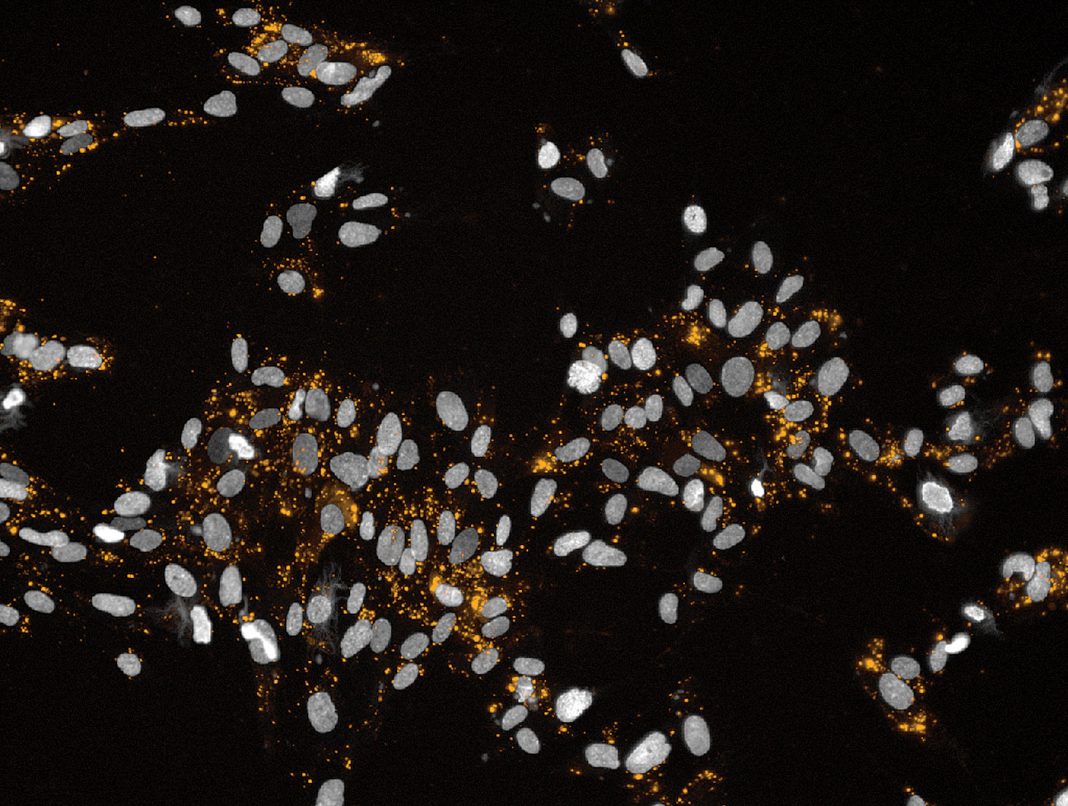
“Eighty-five percent of proteins,” he explains, “have a signature tRNA pair that repeats with high frequency in their genomic sequence, but that are rare in the background genome.” Anima labels the signature tRNA pair with energy-donor or -acceptor fluorescent tags. When the two tRNAs come close together in the ribosome during protein translation, a light pulse is emitted. Scientists can see, in real time, where, when, and how much of the protein is synthesized by ribosomes. Screening for small molecule drugs that increase or decrease the light thus screens for protein translation.
Anima’s platform is segmented into discovery, hit validation/de-risking, and hit optimization. Compound libraries are screened to generate millions of images showing the effect of various compounds on the translation of the target protein. Active compounds are automatically identified. Results are validated using Anima’s global translation and target-specific assays to analyze the compounds’ effects on overall protein synthesis and identify selective hits. The platform can then focus on either specific proteins or larger protein groups or pathways. Hit optimization includes target deconvolution and mechanism-of-action analysis.
One decade of research
Anima’s mRNA modulation approach evolved from University of Pennsylvania research that was performed between 2005 and 2014. “We took it out of the university in 2014,” Slonim recalls. “At the time, I wasn’t in biology. I was a software guy. I created several software companies.”
As a serial entrepreneur, he founded ffwd.me, a tech accelerator that helped launch more than 25 diverse companies. He also was co-founder and CTO of Mercury Interactive, helping it grow to $1 billion in annual revenue before it was acquired by HP for $4.5 billion. He then started, as CEO, another company, Identify Software. It grew, and he sold it to BMC, one of the world’s largest software makers.
Eventually, Slonim was approached by Zeev Smilansky, PhD, who became Anima’s co-founder and CSO. “He described an idea that sounded crazy,” Slonim says. Smilansky outlined the fluorescent labeling of tRNA to cause ribosomes to emit light pulses as they assemble protein. This allows researchers to visualize the production of individual proteins by ribosomes in real time. “Their production looks like clouds of light in the Milky Way,” Slonim observes.
The next logical step was to transfect the tRNA into diseased cells. “Once we started to ‘see the light,’ the idea for a drug discovery platform started.”
Anima’s experience running several screening projects shows that the platform can be used to identify compounds that either up- or downregulate target protein translation.
Anima currently has six in-house drug development programs at the preclinical stage. Three inhibit the synthesis of collagen type I for lung fibrosis, liver fibrosis, or scleroderma. The others target unmet needs involving respiratory syncytial virus (by interfering with viral protein synthesis); oncology (by interfering with c-Myc translation); and Huntington’s disease (by monitoring ribosome pausing during mutant Huntingtin protein translation).
Collaborations are part of the strategy
“Big pharma is very cynical of disruptive technology,” Slonim notes. To mitigate the risks inherent with young, breakthrough technologies, potential collaborators typically push for exclusivity and tend to dictate the rules of collaboration. Startups, desperate for validation and cash, generally accept.
But, Slonim cautions, “If your potential partner is getting exclusivity for many years, ensure you’re compensated for that. Price your technology fairly. Don’t give it away.”
Anima can take this position because its technology is proprietary, licensed to the company exclusively. That exclusivity allows multiple revenue-generating collaborations while simultaneously supporting Anima’s own small molecule programs.
In a 2018 deal, Anima entered into an exclusive collaboration with Eli Lilly and Company. This collaboration, which calls for Anima to use its platform to discover translation inhibitors of targets chosen by Lilly, has the potential to generate more than $1 billion in revenues. Currently, it accounts for an approximately $45 million influx of funds, plus milestone payments.
Breaking the validation barrier
As Anima grows, it faces two challenges: the industry’s unfamiliarity with Israeli innovation, and the industry’s unfamiliarity with Anima’s technology.
“We’re a U.S. company, but we do our development in Israel,” Slonim explains. “There is so much innovation going on in Israel, and its scientists are extremely talented, but American companies generally aren’t familiar with Israel as a place to develop innovative drugs.”
Perhaps Anima’s greatest challenge, however, involves educating potential partners in the biology surrounding mRNA and its potential for developing new drugs.
“A lot of pharma companies are looking into mRNA, which is good, but most are in the early stages. They can’t yet see how it works,” Slonim states. “It’s not surprising that these companies aren’t considering the translation machinery as a drug target, as there is little information regarding the selectivity of translation regulation. They see mRNA, they see it’s translated, and they move on.”
Anima, however, is discovering drugs that selectively control the translation machinery. This is a completely different approach, based on the biology of mRNA translation. “Cells make proteins very specifically, individually, and quickly. Our small molecules target the biology that governs translation,” he reiterates.
The company is spreading that message aggressively. During the past two years, the company has attended some 30 conferences and more than 600 face-to-face meetings. It’s also sharing its findings in an upcoming book chapter about mRNA translation and, of course, is continuing to ink deals and talk with the media.
Don’t expect a flurry of announcements, though. “We’re working on more collaborations and have proof of concept on several promising interactions,” Slonim says, “but we don’t announce small steps. We only announce substantial deals.”
Anima Biotech
Location: 75 Clarement Road, Suite 102, Bernardsville, NJ 07924
Phone: 972-72-214-8770 (Israel)
Website: animabiotech.com
Principal: Yochi Slonim, Co-founder and CEO
Number of Employees: 40
Focus: Anima Biotech has developed Translation Control Therapeutics, a platform technology for the discovery of small molecule drugs that control mRNA translation as a new strategy against difficult and undruggable targets in many diseases.