Cancer cells acquire genetic changes that allow them to grow and proliferate unchecked. Researchers at the Dana-Faber Cancer Institute and colleagues have now found another difference between cancer cells and normal cells. The team analyzed public datasets comprising thousands of cancer DNA samples. Their results showed that X chromosome (chrX) inactivation (XCI), the process by which one of the two X chromosomes in female (XX) cells is inactivated, can also occur in male cancers. High expression of XIST—the gene responsible for shutting down gene expression on the X chromosome—was found in about 4% of the male cancer samples analyzed. The researchers suggest that future research may ultimately point to new therapeutic targets.
Srinivas Viswanathan, MD, PhD, a cancer geneticist and medical oncologist at the Dana-Farber Cancer Institute, is senior author of the team’s published paper in Cell Systems, titled, “Somatic XIST activation and features of X chromosome inactivation in male human cancers.”
Expression of the noncoding RNA XIST is essential for initiating X chromosome inactivation during early development in female mammals, the authors noted. “In normal females, XIST expression serves to achieve dosage compensation between the sexes by silencing one of the two copies of chrX.” Although XIST is typically considered a female-exclusive transcript, it is also found in males in germ cells, and in individuals with supernumerary X chromosomes. Viswanathan explained, “To balance the expression of genes between the sexes, in normal development, one copy of the female X chromosome is inactivated at random across the human body.
While XIST may be expressed in very early development in all sexes, X inactivation is thought to be a female-specific process later in development. It’s also been shown previously that some female cancer cells may lose the ability to turn off one of their X chromosomes, leading to increased X-linked gene expression. X inactivation has still been studied primarily in female cells.
The authors set out to investigate whether XCI may also occur in cancer cells. “We wanted to know if this process that occurs in normal development goes awry in genetically unstable male or female cancer cells,” Viswanathan commented.
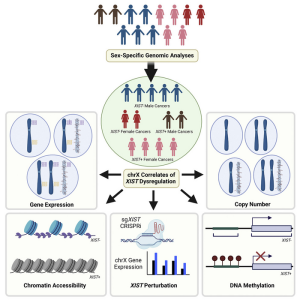
Within the 4% of anomalous male cancer samples identified, 74% were from reproductive cancers already shown to inactivate the X chromosome, but that left 26% of samples from other cancer types. These included liver, brain, skin, heart, lung, and thyroid cancers. “Our results reveal that XIST is expressed and active across a broad spectrum of male cancers,” the investigators stated. “Some of these cancers display hallmarks of XCI, including silencing of gene expression, reduced chromatin accessibility, and increased DNA methylation across chromosome X, suggesting that the developmentally restricted, female-specific program of XCI can be somatically accessed in male cancers.”
“We were very surprised by this result since XIST is a transcript typically used to classify female cancers, and so we wanted to ensure that this was not merely a result of mis-annotation. Yet, we do in fact see that some male cancers of diverse subtypes activate XIST and display features of X inactivation,” said Viswanathan.
“We have to be aware of the caveats of working with these types of datasets. These samples have been in many people’s hands, and there is more room for human error,” said co-corresponding author Cheng-Zhong Zhang, PhD, cancer biologist at the Dana-Farber Cancer Institute. “This is the biggest source of uncertainty for us; we have to be creative in how we look at the data and find controls.”
One possible explanation for why this phenomenon is occurring is genetic instability. Cancers often have multiple copies of chromosomes, and if two X chromosomes happen to be in one cell, then it may be necessary to inactivate one of them by activating XIST, regardless of whether that cell is in a female or male individual.
“Another possibility is there are some important genes on the X chromosome that, when silenced, enable the cancer to grow. We will investigate this in future studies,” said Viswanathan. “In some ways, sex is the ultimate biomarker in that it subdivides the human population, but we often don’t think about how genetic differences between the sexes may inform cancer prognosis or response to therapy.”
In their report, the investigators concluded, “Further studies of the signals that induce XIST expression in certain cancers, the differences between physiological and cancer-related XCI, and the functional consequences for the fitness of cancer cells may illuminate potential targets for therapeutic intervention.”


