May 1, 2017 (Vol. 37, No. 9)
Near-Patient Neuroscience Technology from Cambridge Cognition Unleashes Rich Data Flows
Digital health solutions are improving clinical trials. With these solutions, drug developers can reduce opportunities for human error and increase access to real-world patient data.
Drug developers can also implement on-the-fly cognition testing, provided they use a digital health solution like the one that has been introduced by Cambridge Cognition. This solution, the Cambridge Neuropsychological Test Automated Battery (CANTAB), is a cloud platform that allows researchers to fulfill the following tasks:
- Assess a patient’s mental health in real-world conditions.
- Correlate measures of mental and physical health.
- Access more test data between formal cognitive assessments.
In the past, such capabilities would have been possible only if researchers operated or supervised the use of laboratory-grade equipment. Now, however, patients may be outfitted with wearable, consumer-grade devices.
Cognitive Testing via Wearables
In late February, CognitionKit (a joint venture between Cambridge Cognition and Ctrl Group) partnered with Takeda Pharmaceuticals USA to evaluate how well a wearable technology monitors and assesses cognitive function in patients with major depressive disorder (MDD). The wearable technology incorporates the Apple Watch and the Cognition Kit, an app that combines microcognitive assessments with data from physiological sensors and patient-reported measures of mood.
The partnership calls for a 30-person pilot study and represents Cambridge Cognition’s first significant contract delivering its cognitive tests via wearables rather than in a laboratory or clinic. The study is designed to evaluate the feasibility of using wearables in cognitive testing, gauge compliance with reminders to test, and compare this testing method to traditional neuropsychological testing and patient-reported assessments. Understanding changes in cognition during patients’ daily lives rather than during laboratory visits is expected to maximize patient engagement and help researchers develop more effective, patient-friendly treatments. Results are expected midyear.
In addition to wearable and laboratory-based cognitive testing, Cambridge Cognition also offers an online alternative. The widely validated cloud-based CANTAB Connect platform is used globally to assess cognitive outcomes in clinical trials and academic research projects.
The software’s use has been confined to laboratory sites under the supervision of study staff. But now that the software enables online testing, researchers may assess participants remotely via the web. Because online testing lacks the degree of control possible in the laboratory, certain types of tests—such as those gauging reaction times—aren’t recommended. They can be skewed by differences in hardware and screen response times. Tests of memory, attention, mood, and strategic thinking, in contrast, are as effective online as in the laboratory.
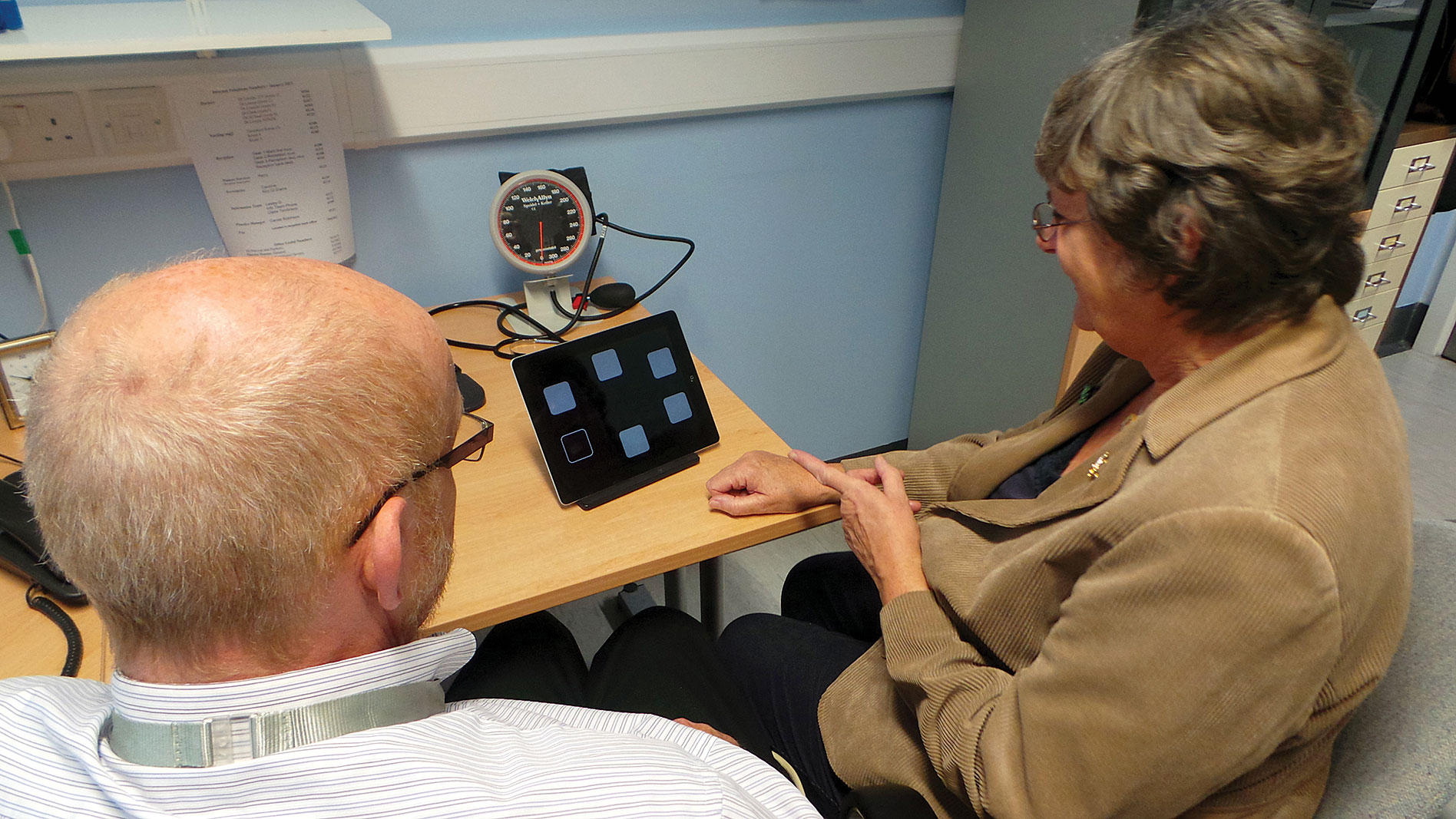
Cambridge Cognition’s digital system for monitoring patient health, the Cambridge Neuro-psychological Test Automated Battery (CANTAB), is available on a mobile platform. This platform, CANTAB Mobile, provides a sensitive and specific touchscreen assessment of episodic memory, mood, and the ability to perform normal daily activities. It is being used to distinguish patients with clinically relevant memory problems from people with normal memory performance after a single doctor’s visit.
Digitization Eliminates Subjectivity
The goal in digitizing cognitive testing is “to replace error-prone and subjective human interactions with automated and objective methods,” says Jenny Barnett, Ph.D., CSO at Cambridge Cognition. “Digitization reduces one source of noise.”
For drug developers, the solution’s expansion and automation capabilities make it easier to translate studies from preclinical to clinical trials and from single laboratories to global studies by ensuring that instructions and scoring for CANTAB are uniform. As a result, human subjectivity and errors are eliminated. From the start, the primarily visual nature of the CANTAB tests ensures that evaluations are culturally and linguistically neutral.
“We provide some of the most sensitive tests on the market for researching the cognitive processes associated with Alzheimer’s disease,” Dr. Barnett tells GEN. CANTAB also can pinpoint cognitive deficits and measure changes over time in other neurological diseases, as well as in depression and other psychiatric conditions.
“For any given CANTAB test, we know whether to expect a given class of drug to have a good or bad effect, and we can identify the parts of the brain involved. These tests measure the activity of precise neural circuits, rather than the clinical picture of a patient,” she explains. More than 2,000 peer-reviewed scientific papers and more than 100,000 citations have been published involving CANTAB.
To ensure that data collected online is of the highest quality possible, Cambridge Cognition conducts frequent technical and scientific validation studies to ensure strong correlations between data collected in the laboratory and that obtained remotely and transmitted online. Software includes standardized voiceover instructions, which guide participants through their assessments, and periodic performance observation checks, which monitor compliance and help ensure that online data is consistent and reliable.
Collect More Data, Inexpensively
“The development of wearables and near-patient technologies means we can capture patient data more frequently and between formal site visits, and do so in a way that represents patients’ real worlds,” asserts Dr. Barnett. “There’s a place for tightly controlled, in-laboratory cognitive testing, but wearable digital devices let researchers collect more data less expensively. Therefore, they can make decisions earlier in the drug development cycle.”
The CANTAB platform can be used in patient recruitment, safety, efficacy, and post-marketing studies. “Drug developers are looking ever earlier in the onset of diseases such as Alzheimer’s, to identify those with very few symptoms for potentially early interventions,” Dr. Barnett elaborates. “These people score below average on memory tests, but above the range of those with easily identifiable symptoms. Adding a 10-minute CANTAB screening to a clinical trial recruitment site, therefore, can eliminate both the ‘worried well’ and those with too much impairment.”
In later-stage trials, CANTAB can be used for long-term, broad-brush characterizations of brain function. Findings are accepted by the FDA as endpoints and can provide the objective evidence regulators want when comparing placebo and drug trials in open-label studies. Amgen, for example, recently used CANTAB in a large Phase III trial to demonstrate that the cognitive effects of taking Repatha® (evolocumab), a cholesterol-lowering drug, are comparable to those of taking a placebo.
In efficacy studies of drugs to improve cognition, CANTAB results are used as a primary metric to determine outcome. “In schizophrenia,” Dr. Barnett says, “when symptoms such as delusion and hallucinations are under control, patients’ major functional issues are cognitive, such as memory problems and inability to concentrate.” Therefore, cognitive functions are logical metrics for drugs being developed for schizophrenia and similar conditions. “There are quite a few disorders in which cognition is impaired.”
The company’s neuroanalytics service provides consulting across disorders and type of trials. “We’re experts in helping with protocols, as well as with data analysis and interpretation,” notes Dr. Barnett.
“As companies move from Phase I through Phase III, there are additional sorts of data they may want to capture,” Dr. Barnett points out. “Instead of a primary endpoint, this data may provide real-world insights on other aspects of health and utility that may help drug developers better understand product differentiation and plan marketing activities. It’s far more effective and efficient to capture long periods of data digitally, through wearables.”
Companies developing therapeutics for neurologic or psychiatric disorders are natural markets for Cambridge Cognition, as well as those developing medications for chronic conditions. “When you are administering drugs for a long period of time, cognition is a noninvasive way to monitor potential brain-safety effects,” Dr. Barnett explains.
Mining Additional Data
Cambridge Cognition has been developing cognitive tests since 2002, when it began enhancing the CANTAB platform, which was originally developed in the 1980s. From CANTAB’s original format, the company has developed a range of tests that are simpler to use; automated; and optimized for desktops, tablets, and (most recently) smart watches.
“Now that our tests are running on cloud platforms, we’re focusing on ways to add value based on electronic data capture,” informs Dr. Barnett. “For example, the score may be the same taking a test on a PC or using pencil and paper. With the computer, though, you also know how long it took for the patient to make the correct choice, and you can identify patterns in patients’ errors. The richness of information may give us additional biomarkers relative to brain function.”
Cambridge Cognition
Location: Tunbridge Court, Tunbridge Lane, Bottisham, Cambridge CB25 9TU, United Kingdom
Phone: 44-1223-810-700
Website: www.cambridgecognition.com
Principal: Steven Powell, CEO
Number of Employees: 65
Focus: Cambridge Cognition helps scientists, researchers, and drug developers improve chances of success in drug development and translational research through near-patient neuroscience technology.



