
Vertex Pharmaceuticals said today it has agreed to acquire Semma Therapeutics for $950 million cash, in a deal that would expand the buyer’s pipeline of specialty treatment with Semma’s potentially curative cell therapy for type 1 diabetes.
Semma said its type 1 diabetes therapy is designed to incorporate a pair of advances: the ability to produce large quantities of functional human pancreatic beta cells that restore insulin secretion and ameliorate hypoglycemia in animal models and a novel device that encapsulates and protects these cells from the immune system, enabling durable implantation without the need for ongoing immunosuppressive therapy.
“We see a substantial opportunity to transform the treatment paradigm for type 1 diabetes, a specialty disease cared for by endocrinologists, both by advancing the development and manufacturing of the cells themselves, as well as through the highly innovative cell/device combination,” Jeffrey Leiden, MD, PhD, Vertex’s chairman, president, and CEO, said in a statement. “This acquisition aligns perfectly with our strategy of investing in scientific innovation to create transformative medicines for people with serious diseases in specialty markets.”
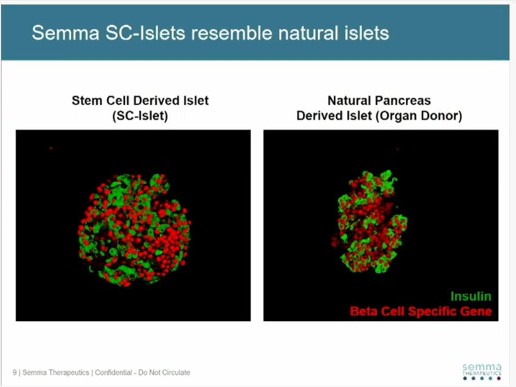
Semma’s treatment is based on a process designed to produce near limitless numbers of stem cell-derived human islets (SC-islets) originating from human pluripotent stem cells. Semma said the SC-islets are functionally equivalent to natural islets from a human pancreas, sensing glucose levels and releasing a precise amount of insulin in response.
SC-islets from Semma’s clinical manufacturing process are more plentiful, reproducible, and robust than purified human pancreatic islets from organ donors. The SC-islets have been transplanted in both small and large animal models of diabetes where they have been shown to survive for more than half a year, the company added.
Semma’s work is intended to advance breakthrough stem cell differentiation technology developed in the laboratory of Douglas A. Melton at Harvard University in 2014 and licensed exclusively to Semma toward generation of cell therapies. Melton is the Xander University professor at Harvard, where he is a co-director of the Harvard Stem Cell Institute, and an investigator of the Howard Hughes Medical Institute.
“Our main challenge is to understand the precursor or stem cells that give rise to the pancreas and to characterize the key gene products that specify cell fates and functions during organogenesis,” Melton’s lab states on its home page. “If our studies are successful, it should be possible to apply our conclusions to human cells and provide a source of insulin-producing beta-cells for diabetics.”
Proof-of-concept presented

On June 29 at the International Society for Stem Cell Research (ISSCR) Annual Meeting 2019 in Los Angeles, Semma’s vp of cell biology research and development, Felicia Pagliuca, PhD, presented data from a pair of preclinical studies showing that Semma had achieved proof-of-concept for two lead programs for type 1 diabetes testing SC-islets in both non-human primates and pigs.
In non-human primates, Semma said at the time, SC-islets were shown to successfully engraft and be highly functional, with the functional cells persisting over six weeks in the non-human primate liver. The transplanted SC-islets reduce the requirement for insulin by more than 60%.
In pigs, Semma continued, SC-islets encapsulated in the company’s immunoprotective device rapidly secreted insulin in response to stimulation quantified by C-peptide, designed to measure beta cell function through insulin production. The device was shown to provide immunoprotection by maximizing cell survival while minimizing foreign body reaction.
“Together these technological advances set the stage for the first clinical tests of stem cell-derived islets,” according to the abstract of Pagliuca’s presentation.
Said David Altshuler, MD, PhD, EVP, global research and CSO: “Their compelling proof-of-concept data in animals demonstrates the opportunity to develop transformative and potentially curative therapies to treat people with type 1 diabetes.”
“Unlike insulin injections and insulin pumps, islet cell transplantation can provide physiologic regulation of blood glucose thereby potentially ameliorating or preventing both the hyperglycemic and hypoglycemic episodes associated with the current standards of care,” Altshuler added.
The board of directors and stockholders of Semma have agreed to the acquisition, Vertex stated in a regulatory filing. Vertex said it will offer any update to its current 2019 financial guidance when it releases third quarter 2019 earnings or upon closing the transaction, whichever occurs later.
Vertex disclosed that it expects to complete the acquisition in the fourth quarter, subject to customary conditions that include expiration or termination of the applicable waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, and the absence of any material adverse effect with respect to Semma.
Upon closing of the deal, privately-held Semma will continue as a wholly-owned subsidiary of Vertex.
“Vertex has a proven track record of serial innovation and a deep commitment to developing transformative therapies for patients in need. Being a part of Vertex will allow the Semma team to rapidly and effectively advance our cell therapy and delivery approaches to patients who need them,” stated Semma president and CEO Bastiano Sanna, PhD.
Sanna will join Vertex as president of Semma, while Melton will continue as chair of Semma’s scientific advisory board and provide oversight and guidance on the research and development of the programs, Vertex said.

