Nanoparticles disguised as human immune cells can enhance the healing powers of a variety of drugs by traveling specifically to the affected cells before they release their cargo of concentrated drugs.
Scientists at the department of nanoengineering and the Moores Cancer Center at the University of California, San Diego (UCSD), have coated drug-filled nanoparticles with the membrane of immune cells and showed that these nanosponges target and deliver drugs to inflamed sites in the lungs where they are needed.
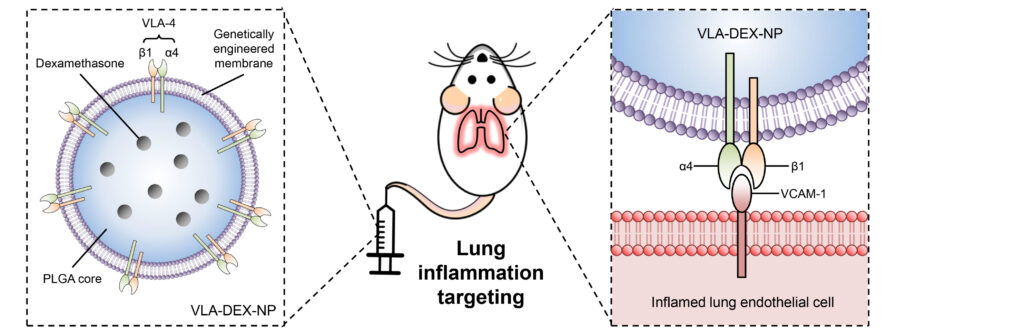
To prove the efficacy of their strategy, the researchers filled the coated nanoparticles with the potent anti-inflammatory drug dexamethasone (DEX) and intravenously injected them in mice with inflamed lungs to show that this treated the inflammation completely at drug concentrations that are not effective through standard delivery methods.
Earlier work in the lab of UCSD nanoengineering professor Liangfang Zhang, PhD, used nanoparticles coated with cell membranes derived from the body’s cells to absorb toxins produced by MRSA, treat sepsis, and target cancer cells.

“In this paper, we used a genetic engineering approach to edit the surface proteins on the cells before we collected the membranes. This significantly advanced our technology by allowing us to precisely overexpress certain functional proteins on the membranes or knockout some undesirable proteins,” said Zhang, senior author on the paper.

The strategy exploits the naturally occurring target-ligand binding affinity between a protein (VACM1) upregulated on inflamed endothelial cells to attract immune cells, and its binding partner on immune leucocytes (VLA-4), to produce the biomimetic nanoparticle capable of targeting inflammation.
“We engineered cell membranes to express the full version of VLA4 all the time,” said Joon Ho Park, PhD, a graduate student in Zhang’s lab and first author on the paper. “These membranes constantly overexpress VLA4 in order to seek out VCAM1 and the site of inflammation. These engineered cell membranes allow the nanoparticle to find the inflamed sites, and then release the drug that’s inside the nanoparticle to treat the specific area of inflammation.”
Nanoparticle delivery allows fast, concentrated, and specific delivery of a drug allowing lower doses to have effects comparable to standard methods of delivery. The authors showed that DEX accumulates at the inflamed lung tissue at higher levels, faster than standard drug delivery approaches.
“We’re delivering the exact same drug used in the clinic, but the difference is we’re concentrating the drugs to the point of interest,” said Park. “By having these nanoparticles target the inflammation site, it means a larger portion of the medicine will wind up where it’s needed, and not be cleared out by the body before it can accumulate and be effective.”
This platform strategy can be applied to treat a variety of diseases caused by local inflammation.
“This is a versatile platform, not just for lung inflammation but any type of inflammation that upregulates VCAM1,” said Park. “This technology can be generalized; this engineered cell membrane-coated nanoparticle doesn’t have to overexpress VLA4, it could be swapped out to another protein that can target other areas of the body or accomplish other goals.”
Park and the team engineered VLA4 overexpressing cell membranes by packaging VLA4 genes into a viral vector and introducing these into lab-grown mouse host cells.
In future studies, the team will examine the process using human cell membranes engineered to express the human version of VLA4.
“By leveraging the established gene editing techniques, this study advances the cell membrane-coated nanoparticles to a new level and opens up new opportunities for targeted drug delivery and other medical applications,” said Zhang.
It may still be a while before the technology can be tested in human clinical trials, but the scientists believe these early results are encouraging.