Ultivue, a developer of tissue biomarker identification and quantification assays for translational and pathology research labs, has completed a $22 million Series C financing.
Proceeds from the financing will go toward expanding its portfolio of UltiMapper™ kits by increasing its level of product customization, Ultivue said.
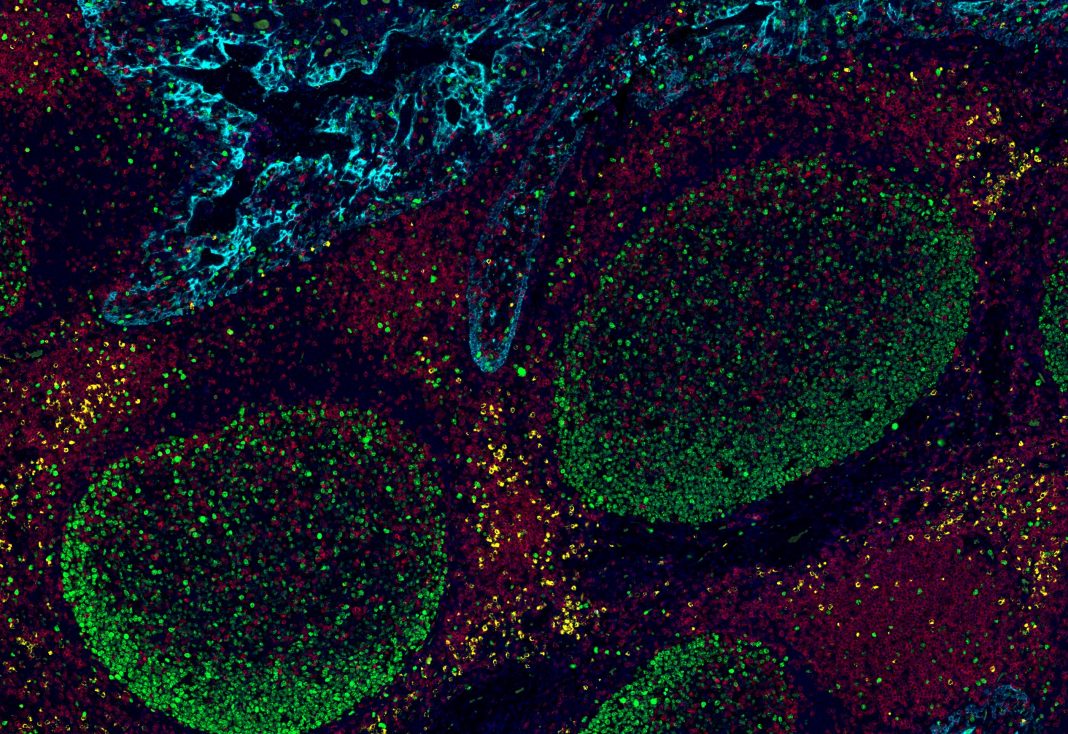
When applied to tissue biopsy samples, Ultivue’s UltiMapper multiplexed assays are designed to enable simultaneous quantitation of multiple biomarkers with sub-cellular spatial resolution, while fitting completely within traditional IHC workflows.
Ultivue plans to expand both its UltiMapper assay product portfolio and its menu of contract research services, with the aim of providing a comprehensive set of personalized medicine solutions for oncology research as well as other therapeutic areas.
Ultivue’s portfolio consist of four 4-plex/5-color research-use-only kits:
- UltiMapper I/O T-act, a T cell activation kit designed to help users determine whether T cells have the potential to mediate cell death by enabling co-expression of proliferating cells, cytotoxic cells, tumor cells, and the underlying proliferative index and potential for T cell mediated death.
- UltiMapper I/O APC, which lets users identify and characterize professional antigen-presenting cells (APCs) by enabling co-expression to visualize specific subsets of cells along the dendritic, monocyte/macrophage, and B cell lineages.
- UltiMapper I/O PD-L1, an immune infiltration kit designed to determine whether a tumor is “hot” or “cold” by enabling co-localization of macrophages, cytotoxic T cells, tumor cells, and the underlying inhibitory or inflammatory mechanisms at play along the PD-L1 axis.
- UltiMapper I/O PD-1, an immune exhaustion kit for designed to enable co-localization of T cells, memory T cells, tumor cells, and the underlying PD-1 checkpoint immune exhaustion mechanism at play.
According to the company, UltiMapper users include translational and clinical researchers seeking to elucidate complex biology and demonstrate the assays’ clinical utility as precision medicine research tools.
Based in Cambridge, MA, Ultivue said it plans a significant expansion of its international market presence across all regions—with an immediate focus on Asia—through increased investments in sales, field application scientists, and marketing.
“This latest round of financing sets the stage for substantial growth and allows us to continue developing the innovation to meet the evolving needs of our customers in translational and clinical research,” Ultivue CEO Michael Natan said in a statement.
The Series C round was led by a new investor, Northpond Ventures, with participation by existing investors that included ARCH Venture Partners, 6 Dimensions Capital, Yonghua Capital, and Applied Ventures.
“We are delighted to receive the backing of Northpond Ventures in addition to our existing investors as we advance the field of multiplex biomarker detection and analysis for tissue phenotyping,” Natan added.
In March, Ultivue signaled its intent to bolster cancer testing when it launched a collaboration with Definiens aimed at expanding Definiens’ standardized IO-Panel capabilities to multiplexed immunofluorescence (IF).
Through their collaboration, whose value was not disclosed, the companies agreed to co-develop a joint offering designed to combine Ultivue’s UltiMapper I/O kits and services with Definiens’ digital image analysis, powered by its Tissue Phenomics platform and supported by leading machine learning technologies.