Stony Brook University researchers are now able to address many long-standing questions about the formation of triglyceride molecules since they were able to visualize the structure of the lipin enzyme, which carries out a critical production step. Findings from this new study—published recently in Nature Communications through an article entitled “Crystal structure of a lipin/Pah phosphatidic acid phosphatase”—will help scientists to better understand how lipins regulate the production of triglycerides. Moreover, the structure also provides scientists with insights as to why mutations in the enzyme cause a loss of activity that leads to abnormal production of triglycerides implicated in heart disease, obesity, and diabetes.
“This structure answers a long-standing question for how two essential regions, N-lip and C-lip, which are located on opposite ends of this protein in humans, come together to form a functional enzyme to help make triglycerides,” explained Mike Airola, PhD, assistant professor in the department of biochemistry & cell biology at Stony Brook University. “Using this structure also helps us understand how the protein interacts with membranes, which is key to regulating its activity and the production of triglycerides.”
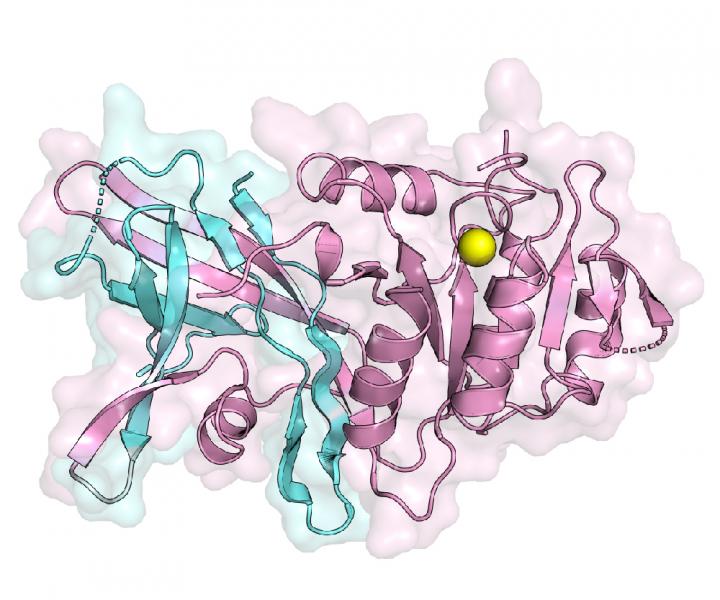
Lipins complete the second to last step of triglyceride production. But when mutations interrupt lipin functions, the body loses its ability to store fat properly, thus potentially triggering a wide variety of metabolic-related conditions. Scientists have unsuccessfully tried to create the first visual structure, a lipin enzyme since these enzymes were identified in 2001. The current study successfully describes the first crystal structure of a specific enzyme called lipin PAP.
“To understand how the N-Lip and C-Lip combine for PAP function, we determined crystal structures of Tetrahymena thermophila Pah2 (Tt Pah2) that directly fuses the N-Lip and C-Lip,” the authors wrote. “Tt Pah2 adopts a two-domain architecture where the N-Lip combines with part of the C-Lip to form an immunoglobulin-like domain, and the remaining C-Lip forms a HAD-like catalytic domain. An N-Lip C-Lip fusion of mouse lipin-2 is catalytically active, which suggests mammalian lipins function with the same domain architecture as Tt Pah2.”
The team used X-ray crystallography, mass spectrometry, and biochemistry to visualize the structure, which represents the active state of a lipin enzyme during the production of triglycerides, as well as its other functions, namely lipoprotein assembly and cellular signaling.
Lead study investigator Valerie Khayyo, a Stony Brook graduate student in the Biochemistry & Structural Biology Program, added that the structure enables researchers to understand and see specific mutational changes in the amino acid building blocks of lipins that result in disease.
Khayyo noted that mutations in lipins are increasingly being identified in patients with muscle disorders as well, such as statin-induced myopathy and extreme cases in childhood rhabdomyolysis.
Overall, Airola says that the structure and study consolidate many previous observations into a unifying framework and sets the stage for scientists to resolve several remaining important questions concerning how lipins are regulated.
“This [data] illustrates mechanisms for lipin/Pah PAP function, membrane association, and lipin-related pathologies,” the authors concluded.