
The researchers envision that their synthetic receptor will be used for the development of practical, ultrasensitive analytical devices for steroid sex hormones, ranging from medical tools to doping controls in sports, in the near future.
The study (“A polyaromatic receptor with high androgen affinity”), published in Science Advances, represents an example of biomimetic design, i.e., the creation of systems that mimic ideas from nature, according to the team. “Natural biological receptors can recognize tiny structural differences between male and female steroid hormones using their protein pockets,” the authors said. “However, it has been challenging to emulate this function artificially until now.”
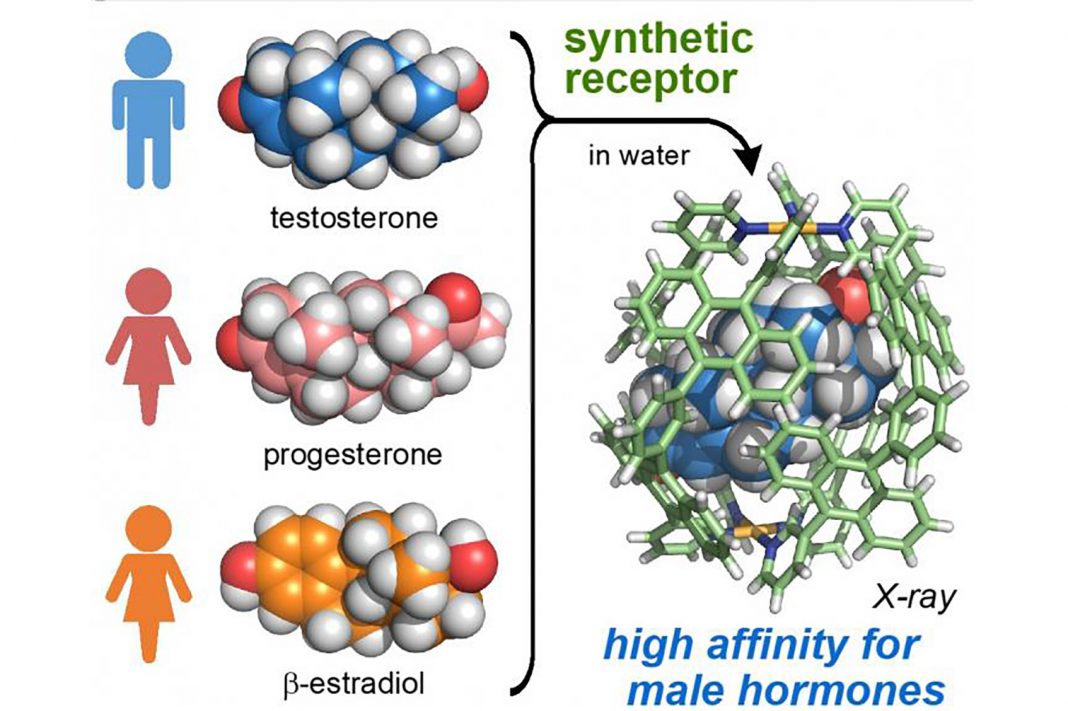
“Biological receptors distinguish and bind steroid sex hormones, e.g., androgen-, progestogen-, and estrogen-type hormones, with high selectivity. To date, artificial molecular receptors have been unable to discriminate between these classes of biosubstrates. Here, we report that an artificial polyaromatic receptor preferentially binds a single molecule of androgenic hormones, known as ‘male’ hormones (indicated with m), over progestogens and estrogens, known as ‘female’ hormones (indicated with f) in water,” the investigators wrote.
“Competitive experiments established the binding selectivity of the synthetic receptor for various sex hormones to be testosterone (m) > androsterone (m) >> progesterone (f) > β-estradiol (f) > pregnenolone (f) > estriol (f). These bindings are driven by the hydrophobic effect, and the observed selectivity arises from multiple CH-π contacts and hydrogen-bonding interactions in the semirigid polyaromatic cavity. Furthermore, micromolar fluorescence detection of androgen was demonstrated using the receptor containing a fluorescent dye in water.”
The researchers note that the key to their advance was the unique design of the cavity (mimicking the natural pocket but using unnatural components) within the receptor. This cavity, encircled by polyaromatic frameworks held together with metal ions, enabled the receptor to act as a semi-rigid container, one flexible enough to complement the shape of the hormone and to induce effective bonding interactions.
The study, conducted by Michito Yoshizawa, PhD, Masahiro Yamashina, PhD, and co-workers, is a continuation of the team’s previous work on developing nanocapsules for a wide range of biosensing applications in the medical and environmental fields. Their experiments showed that the synthetic receptor preferentially binds steroid sex hormones in an order similar to natural androgen receptors, beginning with male hormones such as testosterone and androsterone, followed by female hormones such as progesterone and beta-estradiol. When placed in a mixture of male and female hormones suspended in an aqueous solution at 60 degrees Celsius for ten minutes, the receptor exclusively bound testosterone with more than 98% selectivity. This high level of selectivity was achieved even when the mixture contained a large excess of female hormones.
Using x-ray crystallographic analysis, the researchers observed that the spherical cavity is distorted into an elliptical shape upon encapsulation of testosterone. They say that this conformational change contributes to the enhancement of intermolecular interactions between the receptor and the hormone.
The team also devised a way of using the receptor to detect extremely small amounts of a male hormone. They prepared a receptor-dye complex that emits bluish green fluorescence without testosterone. By adding a nanogram amount of testosterone, the fluorescence decreased considerably upon the encapsulation, representing a new ultrasensitive detection method, the researchers explained.


