Ordinarily, the chaperone protein known as glucose-regulated protein 94 (GRP94) keeps cells on the straight and narrow by ensuring that their proteins fold properly. But when the chaperone undergoes a process called glycosylation, picking up a sugar molecule, it leads cells astray, disrupting protein networks and giving rise to serious diseases such as cancer and Alzheimer’s.
Fortunately, it looks as though “bad” GRP94—that is, GRP94 that has been deranged by its attachment to sugar—could be inhibited by drugs that ignore “good” GRP94. A prototype drug called PU-WS13 has already been identified.
GRP94’s aberrant ways and PU-WS13’s norm-restoring ways have been revealed by researchers at the Memorial Sloan Kettering Cancer Center. These researchers, led by Gabriela Chiosis, PhD, published their findings June 30 in Cell Reports, in an article titled, “Molecular Stressors Engender Protein Connectivity Dysfunction through Aberrant N-Glycosylation of a Chaperone.” The article explores the relationships between stress, the remodeling of the proteome, and the development of various diseases. The remodeling of the proteome has been known to involve chaperone proteins, but the underlying mechanisms have remained obscure. To help clarify matters, Chiosis and colleagues extended work that they performed on chaperones previously.
“Our earlier work showed that defects in chaperones could lead to widespread changes in cells, but no one knew exactly how it happened,” said Chiosis, the current study’s senior author. “This paper finally gets into the nuts and bolts of that biochemical mechanism. I think it’s a pretty big leap forward.”
In the current study, Chiosis and colleagues focused on how GRP94 regulates disease-associated cellular stress. Specifically, the researchers looked at changes in GRP94 in cancer cells, including cells from patients treated for breast cancer at Memorial Sloan Kettering. The researchers found that when GRP94 undergoes a process called glycosylation, in which a sugar molecule is added, it completely changes the way that chaperone behaves.
“It goes from protein that was very floppy and flexible to one that’s very rigid,” explained Chiosis. “This one change is enough to convert it from a good guy in the cell to a bad guy. That, in turn, can make the cell behave in a way that’s not normal.”
“Our study unveils a specific N-glycosylation pattern used by a chaperone, GRP94, to alter its conformational fitness and stabilize a state most permissive for stable interactions with proteins at the plasma membrane,” the authors of the Cell Reports article wrote. “This ‘protein assembly mutation’ remodels protein networks and properties of the cell.”
Essentially, when GRP94 undergoes glycosylation, it moves to a different part of the cell. Normally, it’s found in the endoplasmic reticulum, where proteins are made and folded. But after the sugar is added, it moves to another part of the cell, the plasma membrane. This leads to widespread dysfunction of proteins and a more aggressive cancer.
The N-glycosylated GRP94 variant, Chiosis and colleagues demonstrated, is a small and distinct fraction of the GRP94 pool. Moreover, the scientists found that the proteome dysfunctions mediated by the N-glycosylated GRP94 variant are actionable.
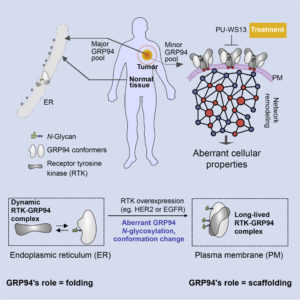
“We show in cells, human specimens, and mouse xenografts that proteome connectivity is restorable by inhibition of the N-glycosylated GRP94 variant,” the scientists indicated. This inhibition was achieved by PU-WS13, a small molecule that acts on GRP94 in the plasma membrane.
“The changes that we saw only happen in diseased cells, such as cancer cells or those related to Alzheimer’s,” Chiosis noted. “That makes them a good target for therapies because healthy cells are unlikely to be affected.”
Chiosis cautioned, however, that more research is needed before a new drug can be developed based on this approach. “PU-WS13 is just a prototype,” she explained. “It has to be tailored for use in humans. We’re investigating how to make this into something that might work as a drug.”


