As we age, we acquire random mutations in the cells that make up our bodies―a phenomenon called somatic mutation. Hematopoietic stem cells (HSC) that continuously replenish the cells in our bloodstream may acquire somatic mutations. HSCs are the precursors of both the oxygen carrying red blood cells and the immune white blood cells.
When HSCs repeatedly make blood cells with the same mutation, the condition is called clonal hematopoiesis (CH). CH associated with aging, is a risk factor for cancers, cardiovascular disease, stroke, and overall mortality.
Certain mutations drive CH, such as mutation in a gene called DNMT3A. But decades may pass between acquiring a mutation in a gene like DNMT3A, and CH. This suggests some other factor, in addition to these mutations, may contribute to CH.
A new study finds chronic infection compounded with a loss of function in the DNMT3A gene drives CH. These findings are published in the article “Chronic infection drives Dnmt3a-loss-of-function clonal hematopoiesis via IFNγ signaling” in the journal Cell Stem Cell.
The study aimed to find out whether infection exerts selective pressure favoring the expansion of DNMT3A mutant HSCs in chimeric mice. To answer this question, the authors created chimeric DNMT3A-mosaic mice by transplanting bone marrow HSCs with and without DNMT3A into normal (wildtype) mice that were previously subjected to radiation to deplete their own stocks of HCSs. This allowed the researchers to track how each subpopulation of HSC shrinks or grows relative to one another over time when infected for several months with Mycobacterium avium bacteria.
The investigators observed substantial expansion of HSCs without the DNMT3A gene during chronic mycobacterial infection along with a reduction in their ability to differentiate into immune cells in the blood, which is contrary to the behavior of normal HSCs in response to chronic infection.
Compared to normal HSCs, HSCs lacking DNMT3A were more resistant to exhaustion and were less sensitive to stress-induced programed cell death or apoptosis upon chronic infection. These findings show how a minor population of HSCs without DNMT3A could in time, overtake a major population of normal HSCs in the presence of chronic infection.
“Previously, we showed that chronic infection significantly impairs the ability of wild-type HSCs to remain in a quiescent stem cell state. Prolonged exposure to a systemic bacterial infection promoted extensive differentiation of HSCs. While this produced sufficient immune cells to fight the infection, it also reduced the number of bone marrow HSCs by 90%,” says Katherine King, PhD, associate professor at Baylor College of Medicine and Texas Children’s Hospital and senior author on the study.

“In contrast, HSCs in mice lacking DNMT3A gene did not differentiate much. In fact, they underwent self-renewal to produce more HSCs. We undertook the current study to test our prediction that defective differentiation and increased duplication of DNMT3A HSCs allows them to overtake and outcompete normal HSCs when fighting chronic infections or facing long-term inflammatory conditions,” says King.
Several chronic inflammatory diseases such as tuberculosis, hepatitis, herpetic infections, and inflammatory bowel disease trigger the release of interferon gamma (IFNγ)—a cytokine protein released by immune cells in response to an infection. IFNγ initiates a cascade of protective immune responses.
The team reports that compared to wild-type HSCs, DNMT3A-loss of function HSCs exhibited an entirely opposite set of cellular responses and global changes in gene expression patterns in response to IFNγ, which tended toward preserving or even increasing the numbers of stem cells at the expense of mounting an effective response against pathogens.
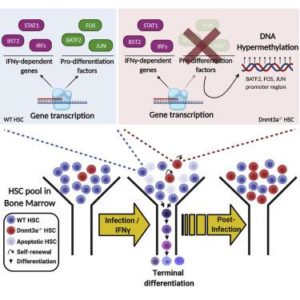
The authors also show that injecting a recombinant IFNγ in mice is sufficient to generate all the attributes (phenotypes) of a loss of DNMT3A in HSCs upon infection —a phenomenon called ‘phenocopying’. The authors profile gene expression and epigenetic changes to show a decrease in HSC differentiation or specialization into red and white blood cells, associated with widespread changes in methylation, and decreased apoptosis, that accounts for clonal expansion of HSCs without DNMT3A during infection.
The authors note, “DNMT3A mutant human HSCs similarly exhibit defective IFNγ-induced differentiation. We thus demonstrate that IFNγ signaling induced during chronic infection can drive DNMT3A-loss-of-function CH.”
“We are excited by the findings of this study which opens several areas of future investigations. We have shown for the first time how chronic inflammation due to long-term infections or autoimmune conditions such as rheumatoid arthritis, ulcerative colitis or Crohn’s disease dampen the body’s immune response as we age. Moreover, it sheds light on the critically important role of DNMT3a in modulating immune responses during chronic infection or stress and also explains how aging and inflammation are linked to blood cancers,” King says.



