There are currently no effective, safe approaches that are suitable for treating acute radiation syndrome (ARS) in mass populations exposed to deadly levels of radiation in the event of a nuclear accident. Studies in mice by researchers at the University of Pittsburgh Medical Center (UPMC) now suggest that stem cells in fat tissue may represent one possible answer. Their research demonstrated, for the first time, that treating animals exposed to high levels of radiation using allogeneic adipose-derived stem cells (ASCs) can mitigate the effects of total body irradiation (TBI)-induced ARS, and increase survival. The findings suggest that stockpiles of these fat-derived stem cells could be used to treat large numbers of people in the event of radioactive emergencies.
“Adipose-derived ASCs have been proven to be safe, they have self-renewal capacity and can undergo differentiation to mature cells,” said research lead J. Peter Rubin, MD, chair of the Department of Plastic Surgery at UPMC. “They also are very easy to propagate in cell culture compared to other cell types, which means they can be stockpiled for application at mass levels in case of radiation accidents.” Rubin and colleagues published the results of their studies in STEM CELLS Translational Medicine, in a paper titled “Allogeneic adipose-derived stem cells mitigate acute radiation syndrome by the rescue of damaged bone marrow cells from apoptosis.”
Nuclear power offers an efficient, reliable way to provide energy to large populations—as long as all goes well. Accidents involving nuclear reactors such as those that took place in 1986 at Chernobyl and at Fukushima Daiichi after the March 2011 tsunami raised major concerns about what would happen in a worst-case scenario, when large numbers of people are simultaneously exposed to high levels of radiation. Total body irradiation (TBI) refers to body exposure to a high radiation dose in a very short timespan, which causes the acute illness known as ARS, which can cause devastating damage to key body systems. “ARS manifestation mainly involves hematopoietic and gastrointestinal organ failure, followed by neurological and other organ damage,” the authors wrote
“In nearly all instances of TBI exposure, the primary life-threatening damage is inflicted on the hematopoietic system, which primarily consists of the bone marrow, spleen, tonsils and lymph nodes involved in the production of blood,” explained study co-author, Asim Ejaz, PhD, from the UPMC Department of Plastic Surgery. “High doses of radiation can cause irreparable damage to the bone marrow, affecting the immune system and potentially causing inflammation and infection.”
A matched hematopoietic stem cell transplant is the current therapy of choice. Patients are either able to donate their own stem cells for transplantation—an autologous transplant— or a donor whose stem cells are a good match is found. But the odds of finding an adequate match is low, at about 30%, and for some populations the odds are even less. “Although bone marrow transplantation is beneficial, maintenance of hematopoietic stem cells, radiation dose determination, and the lack of matched stem cell donor for allogeneic therapy applications are limiting factors,” the authors wrote.
“That fact leads to the major issue with relying on this type of therapy,” Ejaz noted. “In a mass population exposure scenario involving several hundred to millions of individuals, hematopoietic stem cell transfusion is an impossible way forward as there is just no way to treat massive numbers of people who are exposed to radiation at the same time. And unfortunately, such a delay in treatment for a large number of individuals would most certainly lead to an increase in their mortality rate.”
And while several drugs are currently being looked at as potential therapies, none provides complete protection, and all have unwanted side effects, which means there is real need for alternative treatments. “Despite the progress, none of these drugs provide complete protection and their use is associated with side effects, so there is a need to research alternative therapy approaches,” The team continued.
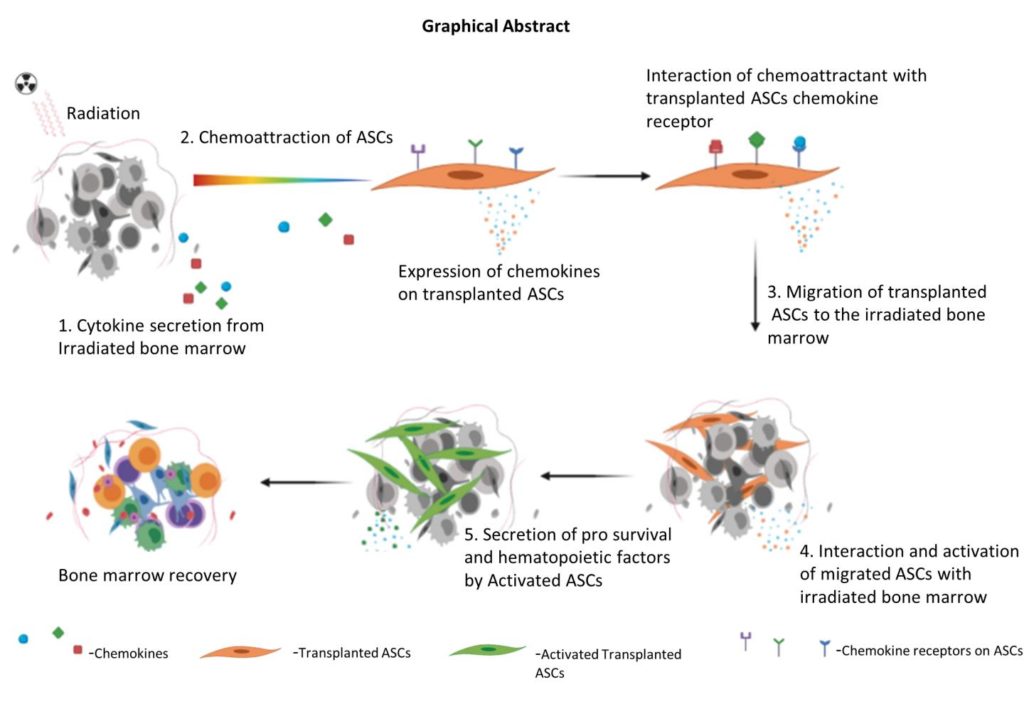
Previous studies have reported that mesenchymal stem cells derived from bone marrow, placenta or Wharton’s jelly can mitigate the effects of ARS. However, low yield, and difficulties in harvesting, and mass production of these cells for stockpiling remain key challenges. The UPMC team has now found that stem cells derived from fat tissue may represent an alternative, “There is one attractive cell-based candidate we’re looking at, and that is adipose-derived allogeneic stem cells (ASCs),” said Rubin “These are mesenchymal stem cells derived from adipose tissue (fat), which can easily be harvested in vast quantities from donors via liposuction.” Because such cells are relatively easy to replicate in cell culture, the potential exists to stockpile them for large-scale use, in the event of radiation accidents.
For their reported studies the scientists compared the effects in mice exposed to high levels of radiation, of treatment using injections of either allogeneic ASCs or autologous ASCs. In particular, they wanted to see if the treatments would improve the animals’ survival rates and repair damage to their hematopoietic systems, when compared radiation-exposed animals that didn’t receive stem cells.
The animals were given the stem cell injections 24 hours after exposure to radiation. When the team then evaluated the effects three days later, they found that the allogeneic ASC-treatment performed as well as autologous cells with respect to improving the animals’ survival and recovery rates, when compared with the non-treated control group. Ejaz noted, “The ASCs had migrated to the bone marrow and facilitated repair by secreting several factors known to reduce oxidative stress and rescue damaged bone marrow cells from apoptosis.”
The authors further noted, “… our results show that intraperitoneally injected allogeneic ASCs migrate to the irradiated bone marrow and mitigate ARS by extending the survival of TBI mice …We show that the mitigation is dependent on ASCs migration to damaged bone marrow and is positively correlated to the survival of damaged cells … Mechanistically, ASCs secrete hematopoietic and prosurvival factors that rescue irradiated damaged bone marrow cells.”
Rubin added, “This suggests that allogeneic ASCs therapy may be beneficial for clinical adaptation to treat TBI-induced toxicities. Further studies will help to advocate the scale-up and adaptation of allogeneic ASCs as the radiation countermeasure.”
As the authors concluded, “We are showing for the first time the use of allogeneic ASCs therapy to mitigate TBI-induced ARS and that intraperitoneally injected ASCs migrate to bone marrow and facilitate repair by secreting hematopoietic and prosurvival factors. This therapy can be conveniently upscaled for a national stockpile storage for mass applications.”



