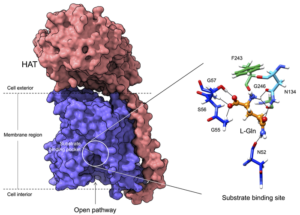
Researchers at the Spanish National Cancer Research Center (CNIO) and the Institute for Research in Biomedicine (IRB Barcelona) have determined the structure of one member of a family of proteins known as heteromeric amino acid transporters (HAT), which transport amino acids across cell membranes. Their studies demonstrated how the structure of, and mutations within HAT family proteins relate to function, and potential involvement in diseases such as cancer and Alzheimer’s disease. The new findings could provide insights that help scientists identify treatments or diagnostic tools for diseases that involve the HAT transporters.
“The combination of structural resolution by cryo-electron microscopy with molecular dynamics calculations and functional studies provides an experimental platform with a lot of potential that allows us to unravel the function of amino acid transporters,” noted Manuel Palacín, PhD, head of the amino acid and disease transporters laboratory at IRB Barcelona, professor at the University of Barcelona, and unit leader of CIBERER. “In this case, we have applied this technology to identify the molecular mechanisms that lead these proteins to transport some amino acids but not others.”
Reporting on their studies in Proceedings of the National Academy of Sciences (PNAS), in a paper titled, “Structural basis for substrate specificity of heteromeric transporters of neutral amino acids,” Palacín, together with first author Carlos Rodriguez, PhD, at the CNIO structural biology program, and colleagues, concluded, “Here, we uncover molecular mechanisms governing substrate specificity within the HAT family of neutral amino acid transporters and provide the structural bases for mutations in LAT2/CD98hc that alter substrate specificity and that are associated with several pathologies.”
Amino acids represent the basic building blocks of life, and play a central role in cellular metabolism, the authors noted. The movement of amino acids into and out of the cell occurs thanks to gates embedded in the cell membrane that are formed by proteins of the HAT family, among others. “The transport of amino acids across the plasma membrane plays a central role in physiology,” the investigators wrote. “… dysregulation of both intra- and extracellular amino acid concentrations is associated with pathological conditions.”
Amino acid transfer across the plasma membrane is mediated by specific transporters that bind and transport these molecules from the extracellular medium into the cell or vice versa. This is the role carried out by members of the HAT protein family. Although HAT proteins are practically identical in structure, some transport certain amino acids, and not others, thereby conferring each member of the family-specific functions, such as the participation in cell growth; a role in diseases such as cancer, neuronal functions, and the transport of toxic substances, and involvement in addiction to substances such as cocaine. Moreover, the scientists commented, “The physiological relevance of HATs is highlighted by their role in cancer and several inherited diseases … Substrate specificity determines the physiological function of each HAT and their role in human diseases.”
HAT transporters of neutral amino acids—designated LAT1/CD98hc, LAT2/CD98hc, and Asc1/CD98hc—in particular have gained scientific interest, as several mutations have been linked to human diseases, the scientists pointed out. Mutations and variations within LAT2/CD98hc, for example, have been linked with age-related hearing loss (ARHL), cataracts, and autism, while overexpression of this HAT has also been linked with chemoresistance in some types of cancer cell, the scientists pointed out.
What confers HAT specificity and diversity of function hasn’t been fully understood, so to try and gain a greater understanding, the scientists applied new techniques to study the 3D structure of HAT proteins. “Classical techniques used to determine the structure of proteins, such as those using X-rays, have had limited success with proteins that are embedded in biological membranes, and so many questions have remained unresolved,” said co-author Oscar Llorca, PhD, head of the macromolecular complexes in DNA damage response group at CNIO, director of the structural biology program of this Center, and an international expert in cryo-electron microscopy. For their studies, the team used this technology, combined with computational modeling and the design of HAT mutants, to observe the structure of one of the members of the HAT protein family, human LAT2/CD98hc—a neutral amino acid transporter—in atomic detail, and decipher its function.
Cryo-electron microscopy allowed the researchers to visualize the protein’s structure at atomic resolution, and determine the pocket where these proteins bind to amino acids, as well as the details of the mechanism by which this recognition occurs. The atomic details revealed that only a few residues of these proteins determine the amino acids to which they bind and therefore their specific functions. In addition, the study demonstrated how the substitutions of some residues for others in these positions in the different members of the family are responsible for modifying the specificity of recognition and transport of some amino acids and not others.
Given the new data, researchers now face the challenge of finding new therapies and diagnostic tools for diseases that involve HAT amino acid transporter proteins, and particularly for those conditions that pose serious health problems, such as cancer and neurodegenerative disorders such as Alzheimer’s disease. The results of this research will now allow efforts to be directed towards compounds that could act on specific regions of these proteins, and to manage the disorders in which they participate.