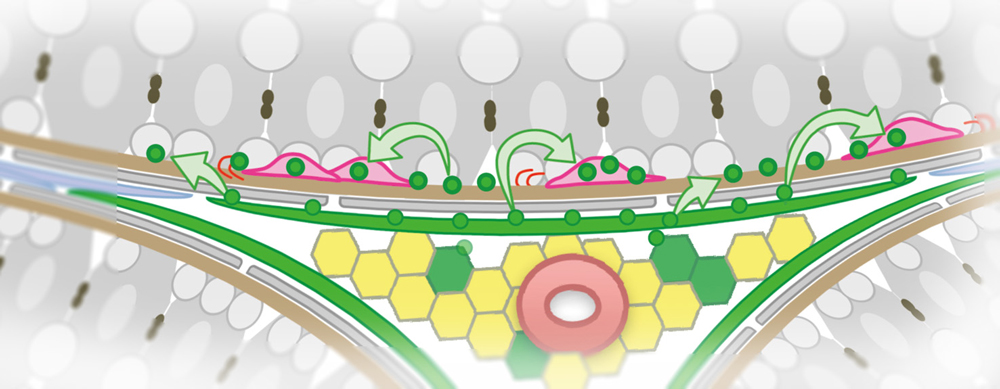
Not all stem cells are confined to stem cell niches, like cows kept in stalls. Instead, some stem cells roam. For example, sperm stem cells mosey across the basement membrane in the testis. Unlike stem cells that huddle within stem cell niches, sperm stem cells lack sustained physical contact with cells that supply self-renewal-promoting factors. And yet sperm stem cells are no less able than niche-dwelling stem cells of maintaining their numbers. The question is, how?
To answer this question, scientists based at the National Institutes of Natural Sciences in Japan studied spermatogenesis in mice. The scientists found that constant sperm stem cell numbers are achieved through a self-organized process in which sperm stem cells actively migrate and compete for a limited supply of self-renewal-promoting fibroblast growth factors (FGFs).
Details appeared December 20 in the journal Cell Stem Cell, in an article titled, “Competition for Mitogens Regulates Spermatogenic Stem Cell Homeostasis in an Open Niche.” In this article, “open niche” doesn’t quite equate with “open range.” Rather, it refers to a sort of pasture, a limited expanse in which sperm stem cells may wander.
“We propose that stem cell homeostasis is achieved through competition for a limited supply of FGFs,” the article’s authors wrote. “We show that the quantitative dependence of stem cell density on FGF dosage, the biased localization of stem cells toward FGF sources, and stem cell dynamics during regeneration following injury can all be predicted and explained within the framework of a minimal theoretical model based on ‘mitogen competition.’”
Quantitative analyses of mice with increased or decreased FGF production revealed a simple mechanism: migratory stem cells uptake and consume FGFs. Stem cells which consume more FGFs are likely to duplicate, while those that consume less are inclined to differentiate. Under this framework, stem cells effectively compete with each other for a limited supply of FGFs, leading the stem cell number to automatically adjust to a particular value, depending on the rate of FGF supply.
This competitive mechanism, the article’s authors suggested, advances our understanding of the regulation of stem cells in tissues without a canonical, anatomically definable stem cell niche—a microenvironment sometimes called an open niche.
“As a general and robust mechanism of stem cell density control, these findings may have important implications for the regulation of stem cell density in other tissue types,” said one of the study’s corresponding authors, Prof. Benjamin Simons, a theoretical physicist at the University of Cambridge.
“Sperm stem cells migrate in the testis to intake FGFs, just as cows move around the meadow to eat the grass which they live on,” added Shosei Yoshida, M.D., Ph.D., the other corresponding author and a developmental biologist at the National Institute for Basic Biology within the National Institutes of Natural Sciences in Japan. “Interestingly, the dynamics of stem cells can be described using mathematics similar to that for an ecosystem.”