When a nerve fiber in the spinal cord is severed, its repair may require that something else be severed: actin. In mature neurons of the central nervous system, actin is stable; that is, actin filaments settle into static arrangements, having long outgrown the need to dynamically reconfigure themselves to accommodate youthful development. These mature neurons can, however, regain the actin-reconfiguring dynamics of youth. And, not at all coincidentally, the mature neurons may again become capable of growing axons, which can, upon reestablishing lost synaptic connections, repair damaged nerves.
Growth-friendly dynamic reconfigurations of actin begin with actin depolymerization, which can be stimulated in mature neurons by upregulating the activity of proteins in the actin depolymerizing factor (ATF)/cofilin family. To test the potential of this upregulating approach in nerve repair, scientists based at the German Center for Neurodegenerative Diseases (DZNE) used mouse and rat models of nerve damage.
In particular, the scientists, led by DZNE’s Frank Bradke, PhD, professor and group leader, experimented with actin dynamics in neurons belonging to the dorsal root ganglion. This is a bundle of neurons that interfaces the spinal cord with the peripheral nervous system.
Following up on recent findings that suggest old neurons can, like developing neurons, regrow after injury, the DZNE scientists focused on morphogenetic transformation at the molecular level. The scientists found molecular mechanisms are shared by mature and developing neurons alike. In mature neurons, however, the mechanisms tend to become rickety with disuse.
These mechanisms, and their possible restoration, were described in a paper (“ADF/Cofilin-Mediated Actin Turnover Promotes Axon Regeneration in the Adult CNS”) that appeared August 7 in the journal Neuron.
“Genetic loss- and gain-of-function experiments followed by time-lapse microscopy, in vivo imaging, and whole-mount analysis show that axon regeneration is fueled by elevated actin turnover,” the article’s authors wrote. “Actin depolymerizing factor (ADF)/cofilin controls actin turnover to sustain axon regeneration after spinal cord injury through its actin-severing activity.”
This finding, which pinpoints ADF/cofilin as a key regulator of axon growth competence irrespective of developmental stage, surprised Bradke. “It is by no means a matter of course that young and adult nerve cells share the same mechanisms,” he said. “Neurons show vigorous growth during embryonic development. Mature nerve cells, on the other hand, usually do not grow and fail to regenerate. Our study now reveals that although the ability to grow is inhibited in adult cells, the neurons keep the disposition for growth and regeneration.”
The scientists noted that cells of the dorsal root ganglion each have two axons: one central, and one peripheral. The peripheral axon can regenerate after damage. It has long been known that the central axon can also regrow; but only if its peripheral counterpart has previously been lesioned. “Why the sequence is like this is still not exactly known,” Bradke said. “We will be looking into this in the future.”
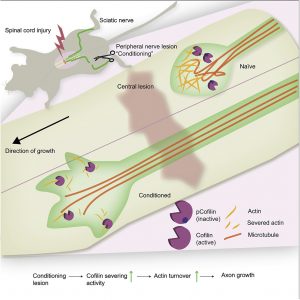
Ordinarily, only neurons of the periphery, that is, neurons in the arms and legs, retain a pronounced potential for mending damaged connections. This potential typically remains unrealized in the central nervous system, where severed axons do not regrow, and disrupted pathways for nerve impulses remain disrupted. Pathway loss can cause paralysis and other serious disabilities.
“For quite some time, we have been wondering whether it is possible to reactivate the processes which manifest in the early developmental phase. This could be a way to trigger regeneration in adult neurons,” said Sebastian Dupraz, PhD, a postdoctoral researcher in Bradke’s lab and a co-author of the current study. “In our recent study, we found that [proteins of the cofilin/ADF family] drive growth and regeneration” not only in developing neurons, but also in adult neurons.
Actin filaments are string-shaped molecules that help build the molecular scaffold that gives the cell its form and stability. The scaffold may be partially dissolved by proteins of the cofilin/ADF family. Only when the scaffold is broken down can neurons change, grow, and regenerate. “An approach for future regenerative interventions could be to target actin,” noted DZNE scientist Barbara Schaffran, another co-author of the current study.
Step by step, the DZNE scientists are trying to understand what makes neurons grow and regenerate. It is a lengthy process. Bradke is, therefore, dampening expectations of rapid progress in the treatment of spinal cord injuries: “We do research in order to set the basis for future therapies. But sadly, you have to be patient until new treatment approaches develop. That’s a long way to go.”



