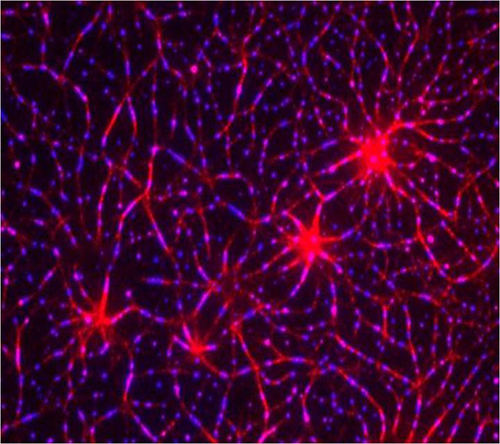
![This is an assembly of a T-cell receptor (TCR) pathway <i>in vitro</i> using 12 purified components on model membranes. An actin network (red) was induced by the adaptor protein LAT (linker for activation of T cells) clustering (blue). The recreation of a TCR signaling pathway independent of the cell itself showed that protein phase separation can create a distinct physical and biochemical compartment that facilitates signaling. [Xiaolei Su]” /><br />
<span class=](https://genengnews.com/wp-content/uploads/2018/08/Apr12_2016_XiaoleiSu_TCellReceptorPathwayInVitro2231167613-1.jpg) This is an assembly of a T-cell receptor (TCR) pathway in vitro using 12 purified components on model membranes. An actin network (red) was induced by the adaptor protein LAT (linker for activation of T cells) clustering (blue). The recreation of a TCR signaling pathway independent of the cell itself showed that protein phase separation can create a distinct physical and biochemical compartment that facilitates signaling. [Xiaolei Su]
This is an assembly of a T-cell receptor (TCR) pathway in vitro using 12 purified components on model membranes. An actin network (red) was induced by the adaptor protein LAT (linker for activation of T cells) clustering (blue). The recreation of a TCR signaling pathway independent of the cell itself showed that protein phase separation can create a distinct physical and biochemical compartment that facilitates signaling. [Xiaolei Su]
T-cell activation without the T cell sounds contradictory, but it is possible, in a limited sense: The signaling pathway that T cells use to respond to threats can be simulated on model membranes. But why do so? According to scientists based at the Marine Biological Laboratory (MBL), recreating a T-cell receptor (TCR) pathway can help reveal how protein signaling works. These scientists report that their simulation efforts have shown that the formation of signal molecule microclusters helps promote T-cell activation.
When a T-cell surface receptor detects a threat, it initiates a chain of signal-protein interactions inside the T cell, interactions that culminate in T-cell activation. These interactions have been known to be accompanied by the formation of microclusters that consist of signal pathway components. Moreover, removing the clusters has been observed to impair signaling. But it hasn’t been clear whether signaling suffered due to the absence of the cluster components or the absence of the clusters themselves.
According to scientists from various institutions who convened at MBL, the clustering matters—protein phase separation can create a distinct physical and biochemical compartment that facilitates signaling. This finding appeared April 7 in Science, in an article entitled, “Phase Separation of Signaling Molecules Promotes T Cell Receptor Signal Transduction.”
The article described how investigators biochemically reconstituted a 12-component signaling pathway on model membranes, beginning with TCR activation and ending with actin assembly.
“When TCR phosphorylation was triggered, downstream signaling proteins spontaneously separated into liquid-like clusters that promoted signaling outputs both in vitro and in human Jurkat T cells,” the authors wrote. “Reconstituted clusters were enriched in kinases but excluded phosphatases and enhanced actin filament assembly by recruiting and organizing actin regulators.”
The investigators noted that although they focused on one TCR signaling pathway involving 12 different proteins, they derived findings that are probably reflective of the way other signaling pathways happen in the cell. The study revealed “a very important self-organization of the protein molecules in the signaling pathway, where they end up clustering to form dense structures in which the proteins are talking to one another,” said co-author Ronald D. Vale, Ph.D., a Howard Hughes Medical Institute (HHMI) investigator at the University of California, San Francisco who is also affiliated with the MBL
The protein molecules separate into structures by a process similar to phase separation of oil and water. According to the Science article, “reconstituted clusters were enriched in kinases but excluded phosphatases and enhanced actin filament assembly by recruiting and organizing actin regulators.” Dr. Vale emphasized that “spatial organization seems to be very important for the efficiency of the signaling pathway.”