The time may have come for wider implementation of chronotherapy, the timed dosing of drugs to follow the daily oscillations of drug targets, which in turn follow the daily oscillations in gene expression. To date, chronotherapy has progressed in fits and starts, mainly because daily oscillations in gene expression have been scrutinized for only a few organs. Now, however, a comprehensive study has appeared. It has produced schedules that capture, for 12 different organs, the 24-hour patterns of expression for thousands of genes.
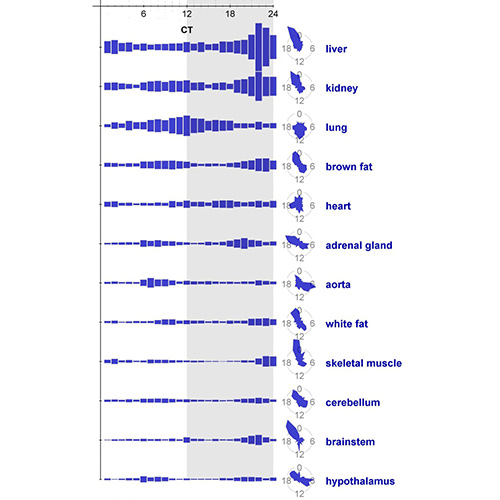
The study was led by John Hogenesch, Ph.D., professor of systems pharmacology and translational therapeutics at the University of Pennsylvania’s Perelman School of Medicine. When Dr. Hogenesch and his research team used RNA-seq and DNA microarrays to quantify the transcriptomes of 12 mouse organs over time, they found that 43% of all protein-coding genes showed circadian rhythms in transcription somewhere in the body, largely in an organ-specific manner. The liver was the most “rhythmic,” having more oscillating genes than any other organ studied.
These results appeared October 27 in the Proceedings of the National Academy of Sciences, in an article entitled, “A circadian gene expression atlas in mammals: Implications for biology and medicine.”
“In most organs, we noticed the expression of many oscillating genes peaked during transcriptional ‘rush hours’ preceding dawn and dusk,” wrote the authors. “Looking at the genomic landscape of rhythmic genes, we saw that they clustered together, were longer, and had more spliceforms than nonoscillating genes. Systems-level analysis revealed intricate rhythmic orchestration of gene pathways throughout the body.”
The rhythmic expression of protein-coding genes was similar to that found for more than 1,000 known and novel noncoding RNAs, including noncoding RNAs conserved between mouse and human.
Of particular interest are the implications of rhythmic gene expression for drug dosing. Most best-selling drugs, the authors found, directly target the products of rhythmic genes. (“Best-selling” status was determined consulting U.S. sales data from Q1 2013 at Drugs.com.) Also, drugs that are deemed essential medicines by the World Health Organization target circadian gene products more often than not.
“Most of these drug targets were not known to be clock-regulated. Many metabolizing enzymes and transporters are, too,” said Dr. Hogenesch. “Because this isn’t appreciated, few of these drugs have been evaluated for time-of-day dependent efficacy, metabolism, or toxicity.”
The study of drug timing has been going on for 40 years and has had several successes such as chemotherapeutics, short-acting statins, and low-dose aspirin. However, many of these studies were done by trial and error.
“Now we know which drug targets are under clock control and where and when they cycle in the body,” asserted Dr. Hogenesch. “This provides an opportunity for prospective chronotherapy.”
Benefits of proper drug timing could include better compliance, improved efficacy, fewer drug-drug interactions, and ultimately, better outcomes at lower costs. To help realize these benefits, Dr. Hogenesch and his team intend to extend their work to specific animal models to look at time of effectiveness. Eventually, though, optimal drug timing will need to be determined via rigorous clinical studies.
In the meantime, investigators interested in advancing chronotherapy may be interested in reviewing the data compiled by Dr. Hogenesch’s team. At a website called CircaDB, researchers can search their favorite gene or find genes that cycle in tissues or organs of interest.