When SARS-CoV-2 infects a host cell, it effectively rewires the host cell. That is, SARS-CoV-2 flips phosphoprotein switches in the host cell’s signaling circuitry to ensure that the host cell supports viral activities such as replication. That infection should entail rewiring is no surprise. What remains hidden are details such as the identity of the switches that get flipped. If these details could be revealed, the rewiring that sustains SARS-CoV-2 infections could be reversed, possibly by drugs that have already been approved for other purposes.
To make sense of host cell signaling—and reveal drug targets (and, potentially, useful drugs)—scientists at Goethe University and the University Hospital Frankfurt studied how SARS-CoV-2 brings about phosphorylation-driven signaling changes in infected human cells. The scientists, led by Goethe University’s Christian Münch, PhD, and University Hospital Frankfurt’s Jindrich Cinatl, PhD, used a comprehensive approach, whole-cell phosphoproteomics.
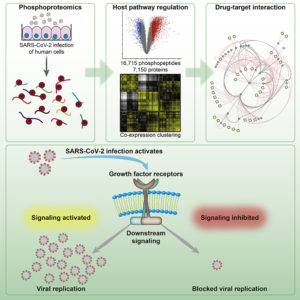
By identifying all the proteins that were carrying a phosphate group at a given moment, the scientists gave themselves the opportunity to recognize which proteins participated in the cell signaling events behind SARS-CoV-2 infections. In fact, the scientists determined that during SARS-CoV-2 infections, growth factor receptor (GFR) signaling and downstream pathways are activated.
This discovery, and its implications, were discussed in an article (“Growth factor receptor signaling inhibition prevents SARS-CoV-2 replication”) that recently appeared in Molecular Cell. Besides identifying the signaling pathways that could be targeted to treat COVID-19, the article noted that the pathways could be interrupted by existing drugs. Several such drugs, which happened to be anticancer drugs, were shown to be effective in cell models of COVID-19.
“Here, we determine changes in the cellular phosphoprotein networks upon infection with SARS-CoV-2 to gain insight into infection-induced signaling events,” the article’s authors wrote. “We found extensive rearrangements of cellular signaling pathways, particularly of GFR signaling.
“Strikingly, inhibiting GFR signaling using prominent (anticancer) drugs—namely pictilisib, omipalisib, RO5126766, lonafarnib, and sorafenib—prevented SARS-CoV-2 replication in vitro, assessed by cytopathic effect and viral RNA replication and release. These compounds prevented replication at clinically achievable concentrations.”
“Due to their clinical availability, these drugs,” the scientists maintained, “could be rapidly transitioned towards clinical trials to test their feasibility as COVID-19 treatment options.”
Phosphate groups play an important biochemical role in the transmission of signals within the cell to stimulate molecular processes that sustain metabolisms or culminate in activities such as cell growth. The phosphate groups are often attached to proteins or removed to control activity. In this process, a change in the protein triggers the next one and the signal is transmitted in a signaling cascade. The target is usually the cell nucleus, where genes are switched on or off.
By conducting a comprehensive survey of phosphate group changes that occur during a SARS-CoV-2 infection, the scientists ascertained which signaling pathways were relevant. Moreover, the scientists could supply mechanistic rationales for how signal-adjusting SARS-CoV-2 drugs might work, rationales that are seldom available if suggestions for signal-adjusting SARS-CoV-2 drugs come from bioinformatic analyses of genetics or cellular data.
“We conducted our experiments on cultivated cells in the laboratory,” added Cinatl. “This means that the results cannot be transferred to humans without further tests. However, from trials with other infectious viruses, we know that viruses often alter signaling pathways in their human host cells and that this is important for virus replication. At the same time, already approved drugs have a gigantic lead in terms of development so that it would be possible—on the basis of our results and just a few more experiments—to start clinical studies very quickly.”



