An increasing number of reports suggest that cardiac arrhythmias are frequent clinical features of COVID-19. A New York-based team of researchers explored the impact of SARS-CoV-2 infection on the pacemaker cells of the heart. To do this, they used both a hamster model and human embryonic stem cell (ESC)–derived sinoatrial node-like pacemaker cells. The team found that SARS-CoV-2 can readily infect pacemaker cells and trigger a process called ferroptosis, in which the cells self-destruct but also produce reactive oxygen molecules that can impact nearby cells. The findings offer a possible explanation for the heart arrhythmias that are commonly observed in patients with SARS-CoV-2 infection.
“This finding suggests that some of the cardiac arrhythmias detected in COVID-19 patients could be caused by ferroptosis damage to the sinoatrial node,” said Robert Schwartz, MD, PhD, associate professor of medicine at Weill Cornell Medicine.
This work is reported in Circulation Research, in the paper, “SARS-CoV-2 Infection Induces Ferroptosis of Sinoatrial Node Pacemaker Cells.”
Arrhythmias including too-quick (tachycardia) and too-slow (bradycardia) heart rhythms have been noted among many COVID-19 patients, and multiple studies have linked these abnormal rhythms to worse COVID-19 outcomes. How SARS-CoV-2 infection could cause such arrhythmias has been unclear, though.
The researchers, including Benjamin tenOever, PhD, professor in the department of microbiology at the NYU Grossman School of Medicine, examined golden hamsters—one of the only lab animals that reliably develop COVID-19-like signs from SARS-CoV-2 infection—and found evidence that following nasal exposure the virus can infect the cells of the natural cardiac pacemaker unit, known as the sinoatrial node.
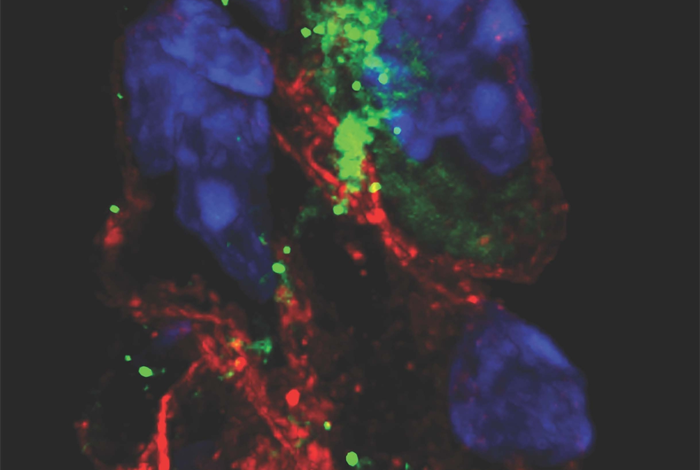
To study SARS-CoV-2’s effects on pacemaker cells in more detail and with human cells, the researchers showed that the induced human pacemaker cells express the receptor ACE2 and other factors SARS-CoV-2 uses to get into cells and are readily infected by SARS-CoV-2. The researchers also observed large increases in inflammatory immune gene activity in the infected cells.
Following exposure to SARS-CoV-2, the researchers performed quantitative real time-PCR, immunostaining, and RNA sequencing to confirm infection and determine the host response of hESC-SAN–like pacemaker cells.
The team’s most surprising finding was that the pacemaker cells, in response to the stress of infection, showed clear signs of a cellular self-destruct process called ferroptosis, which involves accumulation of iron and the runaway production of cell-destroying reactive oxygen molecules. The scientists were able to reverse these signs in the cells using compounds that are known to bind iron and inhibit ferroptosis.
“This is a surprising and apparently unique vulnerability of these cells—we looked at a variety of other human cell types that can be infected by SARS-CoV-2, including even heart muscle cells, but found signs of ferroptosis only in the pacemaker cells,” said Shuibing Chen, PhD, associate professor in the department of surgery and department of biochemistry at Weill Cornell Medical College.
Finally, a high content chemical screen was performed to identify drugs that can inhibit SARS-CoV-2 infection, and block SARS-CoV-2–induced ferroptosis. Two drug candidates, deferoxamine and imatinib, were identified from the high content screen, able to block SARS-CoV-2 infection and infection-associated ferroptosis.
Although in principle COVID-19 patients could be treated with ferroptosis inhibitors specifically to protect sinoatrial node cells, antiviral drugs that block the effects of SARS-CoV-2 infection in all cell types would be preferable, the researchers said.
The researchers plan to continue to use their cell and animal models to investigate sinoatrial node damage in COVID-19—and beyond.
“There are other human sinoatrial arrhythmia syndromes we could model with our platform,” said Todd Evans, MD, professor in surgery at Weill Cornell Medicine. “And, although physicians currently can use an artificial electronic pacemaker to replace the function of a damaged sinoatrial node, there’s the potential here to use sinoatrial cells such as we’ve developed as an alternative, cell-based pacemaker therapy.”