How SARS-CoV-2 infection leads to neurological symptoms, and how the virus gains access to neurons, is unclear. When infecting other cells, the virus binds to the angiotensin-converting enzyme 2 (ACE2) receptor, and enters through endocytosis. However, ACE2 is almost undetectable in the brain.
COVID-19 often leads to neurological symptoms, such as a loss of taste or smell, or cognitive impairments (including memory loss and concentration difficulties), both during the acute phase of the disease and “long COVID.”

This work is published in Science Advances, in the paper, “Tunneling nanotubes provide a route for SARS-CoV-2 spreading.”
Although the human cell receptor ACE2 serves as a gateway for SARS-CoV-2 to enter lung cells—the main target of the virus—and cells in the olfactory epithelium, it is not expressed by neurons. But viral genetic material has been found in the brains of some patients, which explains the neurological symptoms that characterize acute or long COVID. The olfactory mucosa has previously been suggested as a route to the central nervous system, but that does not explain how the virus is able to enter neuronal cells themselves.
According to this new study, SARS-CoV-2 is also thought to be capable of inducing the formation of nanotubes between infected cells and neurons, as well as among neurons, which would explain how the brain is infected from the epithelium.
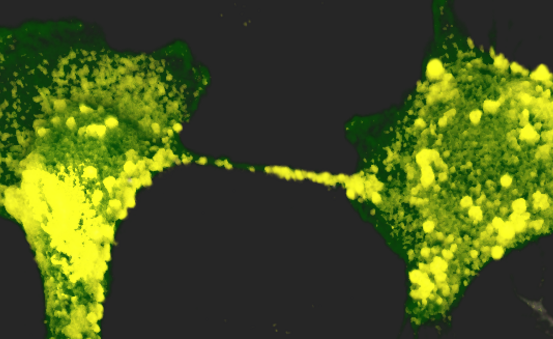
The research team revealed multiple viral particles located both inside and on the surface of nanotubes. Since the virus spreads more rapidly and directly from within nanotubes than by exiting one cell to move to the next via a receptor, this mode of transmission, therefore, contributes to the infectious capacity of SARS-CoV-2 and its spread to neuronal cells. The virus also moves on the external surface of nanotubes, where it can be guided more quickly to cells that express compatible receptors.
“Nanotubes can be seen as tunnels with a road on top,” suggested Chiara Zurzolo, MD, PhD, head of the Institut Pasteur’s Membrane Traffic and Pathogenesis Unit, “which enable the infection of nonpermissive cells like neurons but also facilitate the spread of infection between permissive cells.”
This publication combines research on in vitro cultures, showing that healthy neuronal cells are infected if they come into contact with infected cells, with the use of state-of-the-art microscopy tools. The Titan Krios microscope in the Institut Pasteur’s NanoImaging Core Facility offers unprecedented resolution of biological samples and nanomolecules that is closer to real biological conditions. “With this instrument, novel imaging approaches have been developed to evaluate the structure of SARS-CoV-2 and the architecture of nanotubes,” explained Anna Pepe, PhD, from the Institut Pasteur’s Membrane Traffic and Pathogenesis Unit.
Working in cooperation with the Institut Pasteur’s Ultrastructural BioImaging Core Facility, the research teams used precise investigative methods to detect structures in the nanotubes that were subsequently identified as “virus factories.” The nanotubes between neurons represent a propitious environment for SARS-CoV-2 to develop, since it is invisible to the immune system. Zurzolo believes that “it may represent a mechanism for immune evasion and viral persistence that could be favorable to the virus.”
This study paves the way for further research on the role of cell-to-cell communication in the spread of SARS-CoV-2. It also encourages exploration of alternative therapeutic approaches to hinder the spread of SARS-CoV-2, alongside current projects that are mainly focused on blocking entry via the ACE2 receptor.