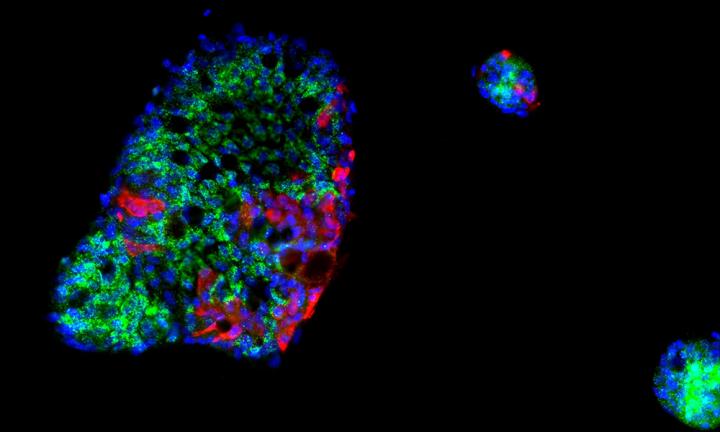
The respiratory tract is given much attention when studying SARS-CoV-2 infection. However, COVID-19 patients show systemic manifestation of the disease in multiple organs. There is clear evidence, for example, of SARS-CoV-2 replication in the gastrointestinal (GI) tract. In an effort to determine the potential for COVID-19 to begin in a person’s gut, and to better understand how human cells respond to SARS-CoV-2, researchers used human intestinal cells to create organoids—3D tissue cultures derived from human cells—which mimic the tissue or organ from which the cells originate. Their single-cell analysis data suggest that SARS-CoV-2 infection can be harbored in a host’s intestines and reveal intricacies in the immune response to SARS-CoV-2.
This study is published in the journal Molecular Systems Biology in a paper titled, “Single-cell analyses reveal SARS-CoV-2 interference with intrinsic immune response in the human gut.”
“Previous research had shown that SARS-CoV-2 can infect the gut,” said Theodore Alexandrov, PhD, team leader at EMBL in Heidelberg, Germany. “However, it remained unclear how intestinal cells mount their immune response to the infection.”
To address this gap, Alexandrov and his colleagues performed single-cell transcriptomics of SARS-CoV-2-infected intestinal organoids. In doing so, they identified a subpopulation of enterocytes as the prime target of SARS-CoV-2 and, interestingly, found the lack of positive correlation between susceptibility to infection and the expression of ACE2.
The researchers were able to determine the cell type most severely infected by the virus, how infected cells trigger an immune response, and that SARS-CoV-2 silences the immune response in infected cells. These findings may shed light on the pathogenesis of SARS-CoV-2 infection in the gut, and indicate why the gut should be considered to fully understand how COVID-19 develops and spreads.
“Interestingly, although most cells in our mini guts had a strong immune response triggered by interferon, SARS-CoV-2-infected cells did not react in the same way and instead presented a strong pro-inflammatory response,” Sergio Triana, lead author and a doctoral candidate in the Alexandrov lab, said. “This suggests that SARS-CoV-2 interferes with the host signaling to disrupt an immune response at the cellular level.”
As the disease progressed in the organoids, the researchers used single-cell RNA sequencing. Among these single-cell technologies, Targeted Perturb-seq (TAP-seq) provided sensitive detection of SARS-CoV-2 in infected organoids. The EMBL research group of Lars Steinmetz, PhD, recently developed TAP-seq, which the researchers combined with powerful computational tools, enabling them to detect, quantify, and compare expression of thousands of genes in single cells within the organoids.
“This finding could offer insights into how SARS-CoV-2 protects itself from the immune system and offer alternative ways to treat it,” Steinmetz said. “Further study can help us understand how the virus grows and the various ways it impacts the human immune system.”