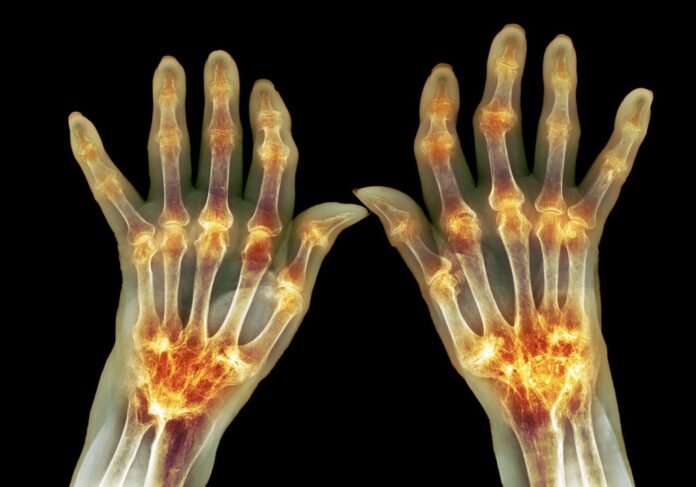
Scientists examining the blood of patients with rheumatoid arthritis (RA) have identified a never-before-seen cell type that could forewarn of worsening symptoms. The RNA sequencing studies, led by Howard Hughes Medical Institute investigator Robert Darnell, PhD, at Rockefeller University, showed that numbers of the cells, dubbed PRIME (pre-inflammation mesenchymal) cells, accumulate in the blood during the week prior to disease flares.
As well as providing new insights that could lead to better prediction of when severe pain and swelling will occur, the newly discovered cells may offer up new insights into the fundamental causes of rheumatoid arthritis, and potentially provide new avenues for treatment, or even preventing flares from occurring. “If we can reliably identify these new cells in patients, we may be able to tell them ‘You’re about to have a flare,’ so they can prepare themselves,” said Darnell. “This would make flares less disruptive and easier to manage.” The team’s published paper in the New England Journal of Medicine is titled, “RNA Identification of PRIME Cells Predicting Rheumatoid Arthritis Flares.”
Rheumatoid arthritis is a disease of the immune system that causes inflammation in the joints, especially around the hands and feet. It can be debilitating and frequently strikes people in their 30s or 40s. The symptoms come in waves, “… with periods of stability interrupted by unpredictable flares of disease activity,” the authors wrote. Current therapeutics, chiefly steroids, can treat these symptoms, but there’s no cure. And while rheumatoid arthritis symptoms can vary in their severity, little is currently known about what causes these symptoms to wax and wane. The researchers pointed out that studies of blood have identified few genes that are significantly associated with rheumatoid arthritis activity.
The Darnell group studies RNA and its connection to diseases such as cancer and neurological disease. For their rheumatoid arthritis studies, the researchers didn’t look directly at the affected joints, but rather examined changes in the blood, leading up to a flare. They used a technique known as longitudinal RNA sequencing—which is a method for analyzing gene expression over long periods of time—to observe any changes during disease states. This technique could feasibly pinpoint molecular variations as a patient’s arthritis symptoms came and went.
To use this approach the lab needed a lot of data. Rheumatoid arthritis patients would normally go to the hospital for monthly bloodwork, but the scientists wanted to see how the blood gene expression patterns changed on a weekly basis. So, rather than having patients come in every week, they developed a finger-prick kit for collecting samples at home. “… we sought to optimize methods by which the patients themselves could obtain fingerstick blood specimens for RNA sequencing (RNA-seq), facilitating weekly blood sampling for periods of months to years,” they wrote.
Over a period of four years, patients mailed their blood samples to the lab and also reported their symptoms, noting when flares occurred. The researchers analyzed blood collected the weeks before symptoms worsened. “Collecting samples longitudinally enabled a search for transcriptional signatures that preceded clinical symptoms, and comparison of these blood RNA profiles with data from synovial single-cell RNA-seq (scRNA-seq) was used to determine whether biologically coherent sets of transcripts were identifiable in the blood before symptom onset and as patients began to have symptoms,” they explained.
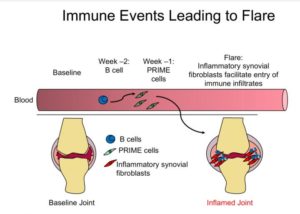
The first observation was not entirely unsurprising. In samples collected two weeks prior to a flare, researchers saw an increase in immune system B cells. That’s not unforeseen, Darnell said, as researchers already know that these cells attacked patients’ joints in rheumatoid arthritis.

But in samples collected just a few days prior to a flare, the scientists noticed an increase in RNA that didn’t match the genetic signature of any known type of blood or immune cell. “That got us thinking there was something fishy going on,” commented study co-author and rheumatologist, Dana Orange, PhD, assistant professor of clinical investigation, in Darnell’s lab.
The cells didn’t look like anything that would normally be found in the bloodstream. Rather, the RNA signature resembled that of bone, cartilage, or muscle cells. “We were so surprised to see that the genes expressed right before a flare are normally active in the bone, muscle, and extracellular matrix—strange pathways to find in blood cells,” said Orange. “That really piqued our interest.”
These new cells, which they dubbed PRIME cells, were normally present only at low levels in the blood, but then spiked in the week before a flare, and all but disappeared during the flare itself. “Longitudinal genomic analysis of rheumatoid arthritis flares revealed PRIME cells in the blood during the period before a flare and suggested a model in which these cells become activated by B cells in the weeks before a flare and subsequently migrate out of the blood into the synovium,” the investigators reported.
The function of PRIME cells remains to be established, but the scientists already have some clues. Based on RNA expression profiles, they found that cells appear strikingly similar to synovial fibroblasts, which are found in the tissue linings of joints. Darnell believes that PRIME cells may be precursors to these fibroblasts, which are known to play a role in causing rheumatoid arthritis symptoms. For example, in mouse experiments where scientists transplanted fibroblasts from an inflamed joint into a healthy joint, the recipient joint would also later became arthritic. “Taken together, our observations suggest a model in which sequential activation of B cells activates PRIME cells just before flares; these cells are then evident during flares in inflamed synovium as inflammatory sublining fibroblasts,” the team stated.
The scientists are now working to learn as much as possible about PRIME cells, and how to quickly detect them. If doctors can easily test for the presence of PRIME cells in the blood, they may be able to prewarn patients of the likelihood of a flare. And if it turns out that PRIME cells play a role in causing the flares, the discovery may also open the door to developing therapies that can reduce inflammation. “For doctors and patients, intervention before a flare up is always better than just treating symptoms,” said Darnell. “If these cells are the antecedents to joint sickness, they become a potential target for new drugs.”
The team’s strategy could feasibly also be used to study waxing and waning in other inflammatory diseases. “Such waxing and waning clinical courses are characteristic of many autoimmune diseases, including multiple sclerosis, systemic lupus erythematosus, and inflammatory bowel disease, underscoring a need to develop approaches to understand the factors that trigger transitions from quiescence to autoimmune flare,” they stated.
One of the team’s next steps is to evaluate in greater numbers of patients whether the presence of these PRIME cells can reproducibly predict a flare, Darnell said. The researchers are still recruiting patients for this study; currently, the team’s blood collection system is only available for use in research. Darnell also wants to study the molecular characteristics of PRIME cells. If the cells are found to play a causal role in flares, he said, “understanding the unique aspects of PRIME cells might enable us to target them with a drug and get rid of them.”