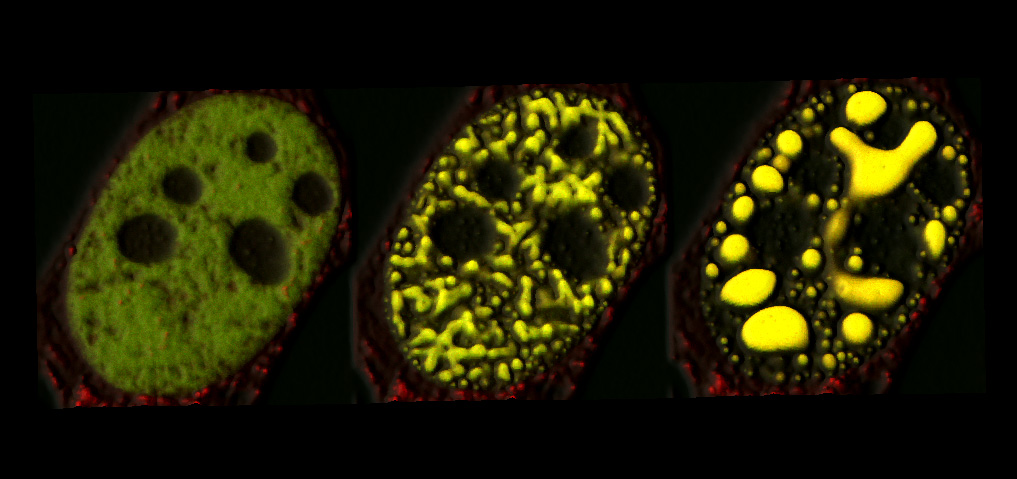
The once-hidden hand known as the membraneless organelle has been caught red-handed. Yes, membraneless organelles have been implicated in diseases including cancer, Alzheimer’s, and amyotrophic lateral sclerosis (ALS). But these cellular compartments, which assemble and disassemble themselves via liquid–liquid phase transitions, have been hard to visualize.
No longer.
Thanks to a team of scientists based at Princeton University, membraneless organelles may be summoned at will, effectively drawn into the open, where they may be exposed to the spotlight of scientific inquiry. The team, led by professor of chemical and biological engineering Clifford P. Brangwynne, Ph.D., has developed a pair of light-controlled techniques that can trigger the formation of membraneless organelles within the cell, at selected cellular locations. One of the techniques, Corelets, may be used to map intracellular phase diagrams, which help reveal the mechanisms that create organelles in some local regions of the cell, but not in others.
The other technique, CasDrop, may be used to scrutinize chromatin. Using CasDrop, the Princeton team found that as membraneless organelles form within the nucleus, they deform the chromatin in unexpected ways. The membraneless organelles push out unwanted genes but can also, at the same time, pull together specifically targeted genes. The droplets can thus function like little, mechanically active machines to restructure the genome.
Details about the two techniques appeared November 29 in the journal Cell. Corelets was the subject of a paper entitled, “Mapping Local and Global Liquid Phase Behavior in Living Cells Using Photo-Oligomerizable Seeds.” CasDrop was described in a paper entitled, “Liquid Nuclear Condensates Mechanically Sense and Restructure the Genome.”
According to Dr. Brangwynne, the findings presented in these papers may be less important, in the long run, than the new tools. “These technology systems,” he said, “should prove to be powerful tools for basic research and have many applications, particularly with regard to human health.”
The Corelets system uses genetically engineered, photosensitive proteins that shapeshift and change their behavior when exposed to light. The proteins, in this case human blood proteins called ferritin, crowd together into a tiny sphere. Exposure to a blue light causes other proteins to stick to the ferritin sphere. By altering certain parameters, the researchers can use the technique to trigger phase separation in different areas of cells.
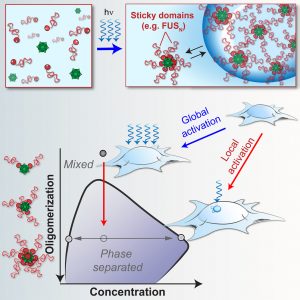
“Surprisingly, both experiments and simulations show that while intracellular concentrations may be insufficient for global phase separation, sequestering protein ligands to slowly diffusing nucleation centers can move the cell into a different region of the phase diagram, resulting in localized phase separation,” noted the authors of the Corelets paper. “This diffusive capture mechanism liberates the cell from the constraints of global protein abundance and is likely exploited to pattern condensates associated with diverse biological processes.”
The CasDrop system builds on CRISPR-Cas9 technology to address particular genes in the cell. Brangwynne and colleagues engineered Cas9 to function as a platform, which upon light activation causes other proteins to bind to the gene, and locally phase separate, forming little dew droplets on the field of chromatin.
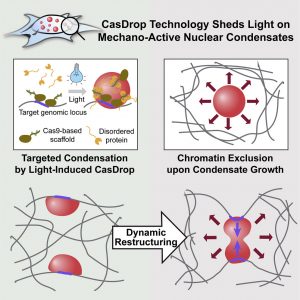
“The CasDrop system enables controlled liquid condensation at specific genomic loci,” the authors of the second paper noted, with intrinsically disordered protein (IDR)-driven condensates “growing preferentially in regions of low chromatin density.” The authors added that nuclear condensates may function as mechano-active chromatin filters, physically pulling in targeted genomic loci while pushing out non-targeted regions of the neighboring genome.
Phillip A. Sharp, Ph.D., a Nobel laureate and professor at the Koch Institute for Integrative Cancer Research at the Massachusetts Institute of Technology, was not involved in the studies, but he commented that the findings are advancing our understanding of membraneless organelles.
“Brangwynne and colleagues have invented a novel method to investigate how interactions between proteins dynamically form condensates with phase transition properties in living cells,” he said. “The two papers highlight exciting discoveries at the interface of physics and cell biology that will lead to new treatments for diseases ranging from cancer to Alzheimer’s.”