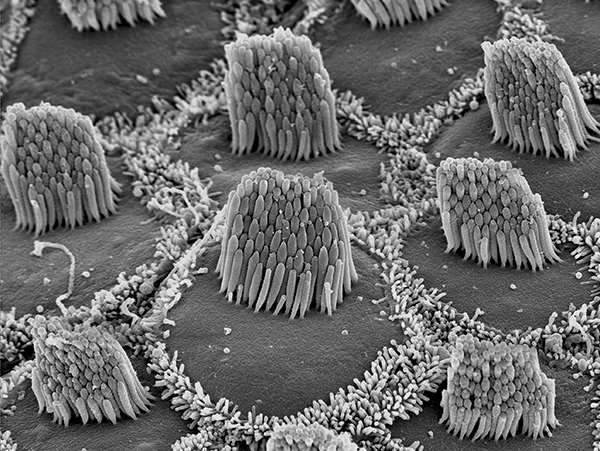
![Findings from this new study have identified a new protein that is essential to the process of turning sound waves into meaningful brain signals. Stereocilia off the inner ear (above). [NIH]](https://genengnews.com/wp-content/uploads/2018/08/bioart2014gillespieandspinelli2474821084-1.png)
Findings from this new study have identified a new protein that is essential to the process of turning sound waves into meaningful brain signals. Stereocilia off the inner ear (above). [NIH]
Nearly 40 million Americans suffer from some level of hearing loss—which includes approximately 74,000 children with profound, early-onset deafness—with almost 50% of these cases stemming from genetic causes. While the cellular architecture and basic biology surrounding human auditory perception have been understood for quite some time, the underlying molecular mechanisms that mediate the conversion of sound waves into signals that the brain interprets as sound is still unclear.
Now, researchers from the University of Maryland School of Medicine (UM SOM) have just published results identifying a crucial protein in the auditory translation process. Findings from the new study were published online today in Nature Communications in an article entitled “CIB2 Interacts with TMC1 and TMC2 and Is Essential for Mechanotransduction in Auditory Hair Cells.”
CIB2, or calcium and integrin-binding protein 2, was found previously to be essential for the structure of stereocilia, the structures at the top of the sensory hair cells in the inner ear. Stereocilia are extremely small, less than a half a micrometer in diameter, which is about the wavelength of visible light. Each ear contains 18,000 hair cells that do not divide or regenerate.
UM SOM investigators discovered five years ago that CIB2 was involved in hearing. Since then, they have studied this protein in flies, mice, zebrafish, and humans. The new study is the first to explain the mechanism behind CIB2 in hearing.
In the current study, the research team genetically engineered mice without CIB2, as well as mice in which a human CIB2 gene mutation had been inserted. The researchers found that the human mutation affects the ability of the CIB2 protein to interact with two other proteins, TMC1 and TMC2, which are crucial in the process of converting sound to electrical signals. This process is known as mechanotransduction.
“We are very excited by these results,” remarked co-senior study investigator, Zubair Ahmed, Ph.D., professor in the department of otorhinolaryngology head & neck surgery at UM SOM. “This tells us something new about the fundamental biology of how hearing works on a molecular level.”
Individuals with the CIB2 mutation cannot turn sound waves into signals that the brain can interpret, leading to deafness. When the researchers inserted the human CIB2 mutation into the mouse, they found that the mice were deaf.
“This is a big step in determining the identity of key components of the molecular machinery that converts sound waves into electrical signals in the inner ear,” explained co-senior study investigator, Gregory Frolenkov, Ph.D., associate professor in the department of physiology at the University of Kentucky.
The researchers are currently looking for other molecules beyond CIB2 that play a key role in the mechanotransduction process. Moreover, they are exploring potential therapies for CIB2-related hearing problems—using the gene-editing tool CRISPR to modify dysfunctional CIB2 genes in mice. They suspect that if this modification occurs in the first few weeks after birth, these mice, which are born deaf, will be able to hear. The scientists are also experimenting with gene therapy, using a harmless virus to deliver a normal copy of the normal CIB2 gene to baby mice that have the mutated version.